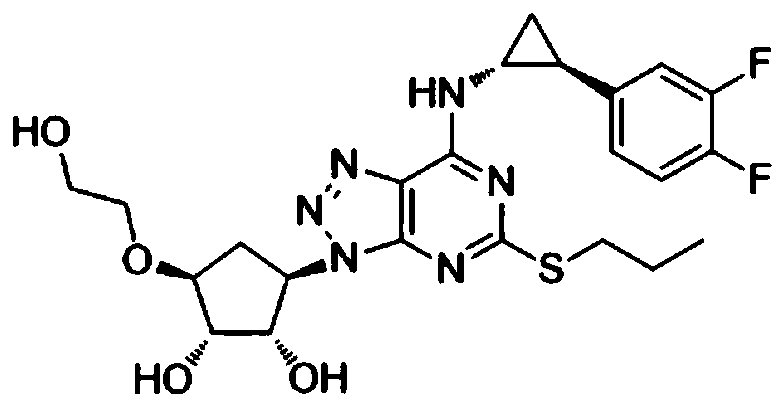
Ticagrelor preparation composition and preparation method thereof
A technology of ticagrelor and a composition, applied in the field of pharmaceutical preparations, can solve the problems of poor fluidity, difficult to solve the problem of rapid disintegration, difficult to realize rapid disintegration of drugs, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
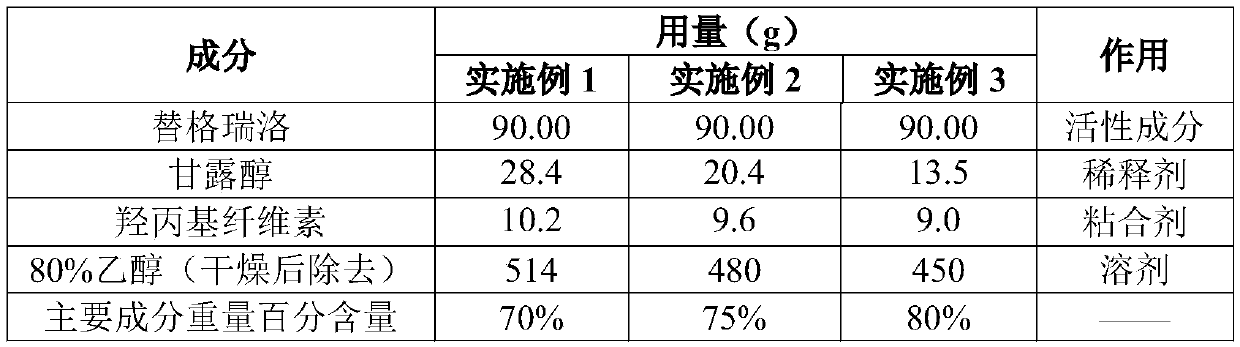
[0046] Embodiment 1~3 preparation of ticagrelor preparation composition
[0047] The first step is the crushing of raw materials.
[0048] The raw material of ticagrelor used in the preparation of the preparation composition of the present invention is the same batch, but it needs to be pulverized before the preparation of the preparation composition of the present invention. The inventor made the batch of raw materials into two kinds of raw materials with different particle sizes by airflow pulverization method , to be distinguished by different sub-batch numbers, wherein, embodiment 1 and 2 adopt the raw material of the 1st sub-batch, and embodiment 3 adopts the raw material of the 2nd sub-batch, and the particle size distribution of the raw material after pulverization is shown in the following table:
[0049] Particle size distribution of ticagrelor raw material after crushing in table 1
[0050] Raw material (crushing) batch
Sub-batch 1
Sub-batch 2
...
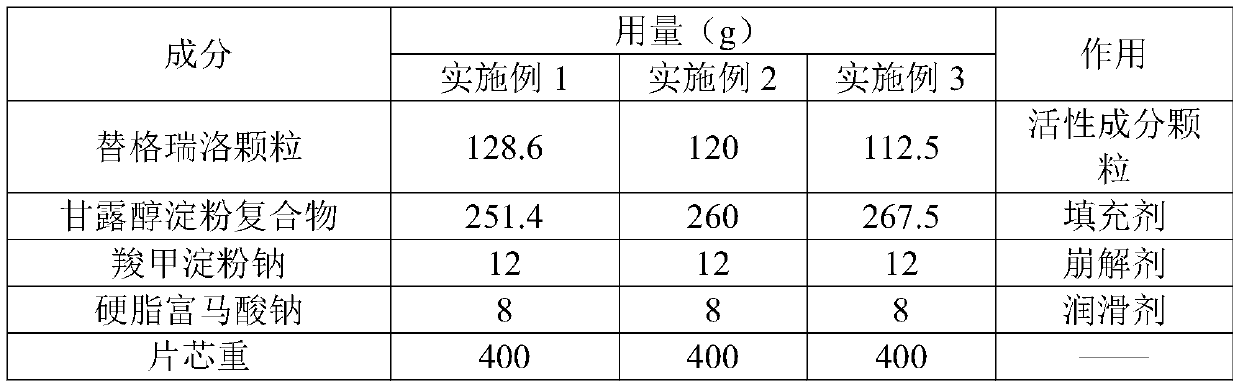
Embodiment 4
[0063] Example 4 Investigation of the powder properties of ticagrelor granules
[0064] In order to investigate the fluidity improvement effect of ticagrelor granules on ticagrelor raw materials, to ensure that the direct compression method successfully prepares a qualified preparation composition suitable for disintegration, the ticagrelor prepared in Examples 1-3 The particles were tested for intermediate control as follows, and the results are shown in the table below.
[0065] Table 4 Powder Science Investigation of Ticagrelor Granules
[0066]
[0067] From the above investigation, it can be seen that after ticagrelor is prepared into ticagrelor granules by spray drying method, its fluidity is obviously improved.
Embodiment 5
[0068] Example 5 Investigation of the powder properties of ticagrelor blended granules
[0069] Table 5 Powder study of ticagrelor blended granules
[0070] Example
Example 1
Example 2
Example 3
0.43
0.41
0.40
Carr Coefficient
12.2%
13.6%
13.4%
33°
33°
33°
[0071] Note:
[0072] Carr index = (tapped density - bulk density) / tapped density * 100%, the range of Carr index: 5-15%, excellent; 12-16%, good; 18-21%, general, can be pressed into tablets; 23-35%, poor; >40%, very poor.
[0073] Particle angle of repose: 25-30° is excellent; 31-35 is good; 36-40° is fair; 41-45° can be compressed; 46-90° is poor.
[0074] From the investigation results of the physical properties of the blended granules in Examples 1 to 3, the blended powder has good fluidity and can meet the requirements of the subsequent tableting process.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com