A controlled-release membrane capable of controlling the release of polypeptide drugs and its preparation method and application
A technology of controlled release membrane and polypeptides, which is applied in the fields of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of lack of controlled release membrane types, small selectivity, and obstacles to the development and utilization of polypeptide drugs, and is conducive to the development of Utilization, simple production process, good batch repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
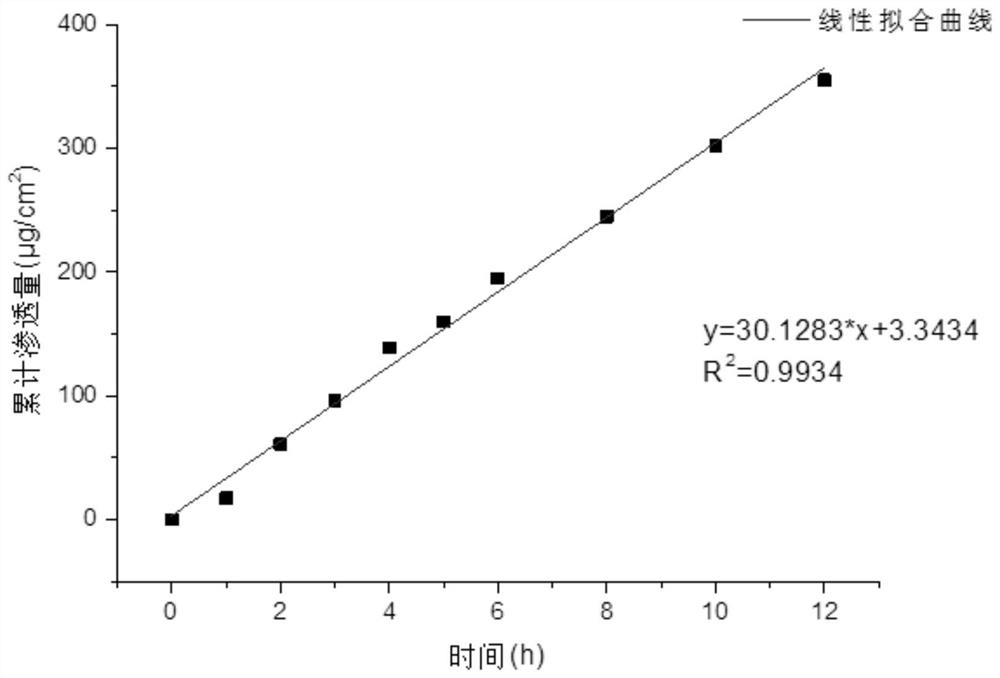
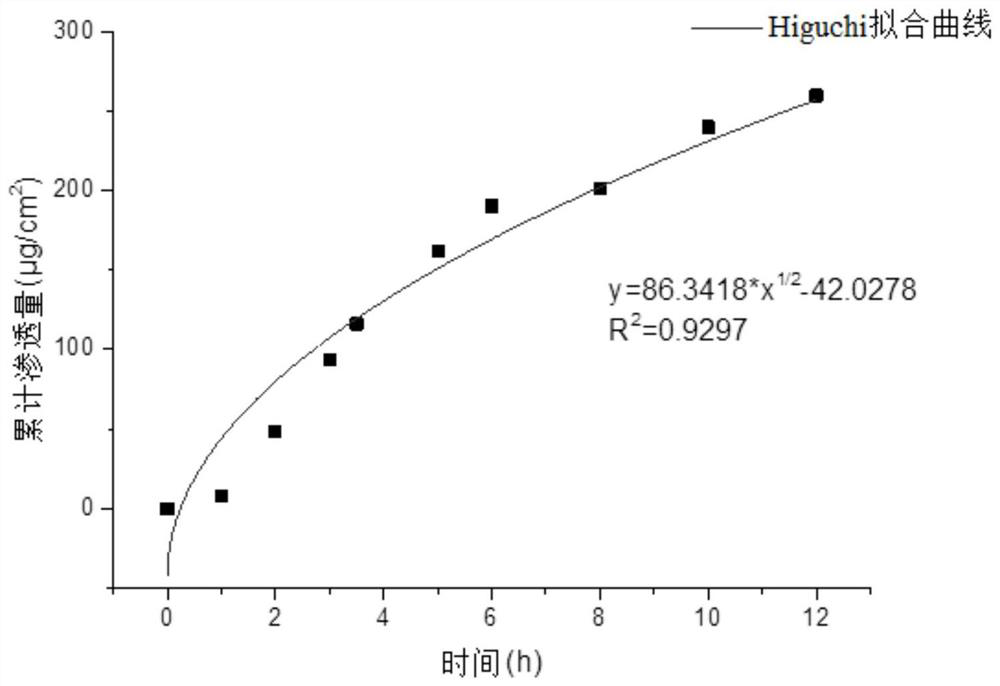
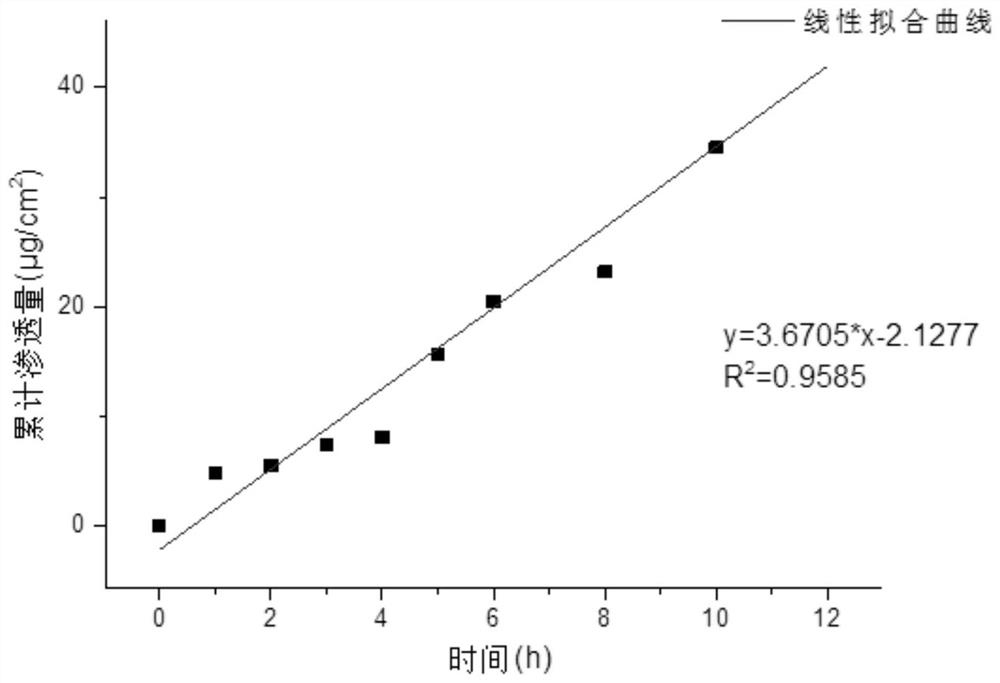
Image
Examples
Embodiment 1
[0041] Preparation of Polyacrylate-Polyethylene Glycol 6000 / 400-MgO Controlled Release Membrane
[0042] Acrylate-(2-hydroxy-3-phenoxy)propyl, acrylate-4-hydroxybutyl, 1,6-hexanediol diacrylate, acrylate-2-(2-(2-ethylhexyloxy Base) ethoxy) ethyl ester was mixed uniformly according to the mass ratio of 9:9:1:1, then 3% benzophenone peroxide was added, stirred and dissolved, and mixed uniformly to obtain mixed solution A. According to the mass ratio of mixed solution A to PEG6000, PEG400, and magnesium oxide particles 82.5:5:5:7.5, accurately weigh PEG6000, PEG400, and magnesium oxide particles (50±10nm), and stir evenly to obtain the final mixture C. Take an appropriate amount of mixture C, evenly spread it in a stainless steel mold with a groove depth of 0.01 mm, and place it in an ultraviolet curing instrument to cure completely to obtain a polymer film. Carefully separate the polymer film from the stainless steel mold, then immerse the polymer film in a hydrochloric acid so...
Embodiment 2
[0044] Preparation of Polyacrylate-Polyethylene Glycol 10000 / 400-MgO Controlled Release Membrane
[0045] Mix acrylate-(2-hydroxy-3-phenoxy)propyl, 4-hydroxybutyl acrylate, and 1,6-hexanediol diacrylate in a mass ratio of 4:4:2, then add 5% Benzophenone peroxide was stirred and dissolved, and mixed uniformly to obtain mixed solution A. According to the mass ratio of mixed solution A to PEG10000, PEG400, and magnesium oxide particles 80:5:5:10, accurately weigh PEG6000, PEG400, and magnesium oxide particles (50±10nm), and stir evenly to obtain the final mixture C. Take an appropriate amount of mixture C, evenly spread it in a stainless steel mold with a groove depth of 0.01 mm, and place it in an ultraviolet curing instrument to cure completely to obtain a polymer film. Carefully separate the polymer film from the stainless steel mold, then immerse the polymer film in a hydrochloric acid solution with a concentration of 1.0 mol / L, and let it stand for 48 hours. After 48 hours...
Embodiment 3
[0047] Preparation of Polyacrylate-Polyethylene Glycol 400-MgO Controlled Release Membrane
[0048] Acrylate-(2-hydroxy-3-phenoxy)propyl, acrylate-4-hydroxybutyl, 1,6-hexanediol diacrylate, acrylate-2-(2-(2-ethylhexyloxy Base) ethoxy) ethyl ester was mixed uniformly according to the mass ratio of 9:9:1:1, then 3% benzophenone peroxide was added, stirred and dissolved, and mixed uniformly to obtain mixed solution A. According to the mass ratio of mixed solution A to PEG400 and magnesium oxide particles 80:5:15, accurately weigh PEG6000, PEG400 and magnesium oxide particles (50±10nm), and stir evenly to obtain the final mixture C. Take an appropriate amount of mixture C, evenly spread it in a stainless steel mold with a groove depth of 0.01 mm, and place it in an ultraviolet curing instrument to cure completely to obtain a polymer film. Carefully separate the polymer film from the stainless steel mold, then immerse the polymer film in a hydrochloric acid solution with a concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com