2, 5-furandicarboxylic acid preparing method
A technology of furandicarboxylic acid and dimethyl sulfoxide, applied in the direction of organic chemistry and the like, can solve the problems of low efficiency and low concentration of reaction substrate, and achieve the effects of high efficiency, easy control of oxidation process and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
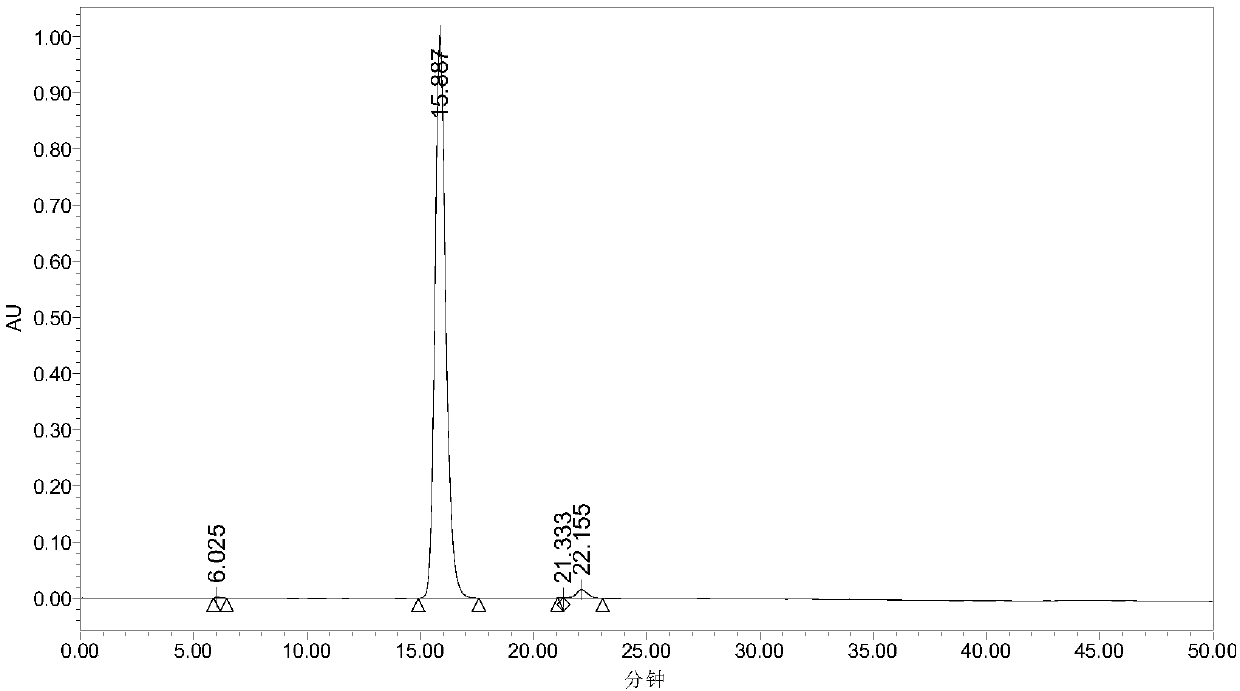
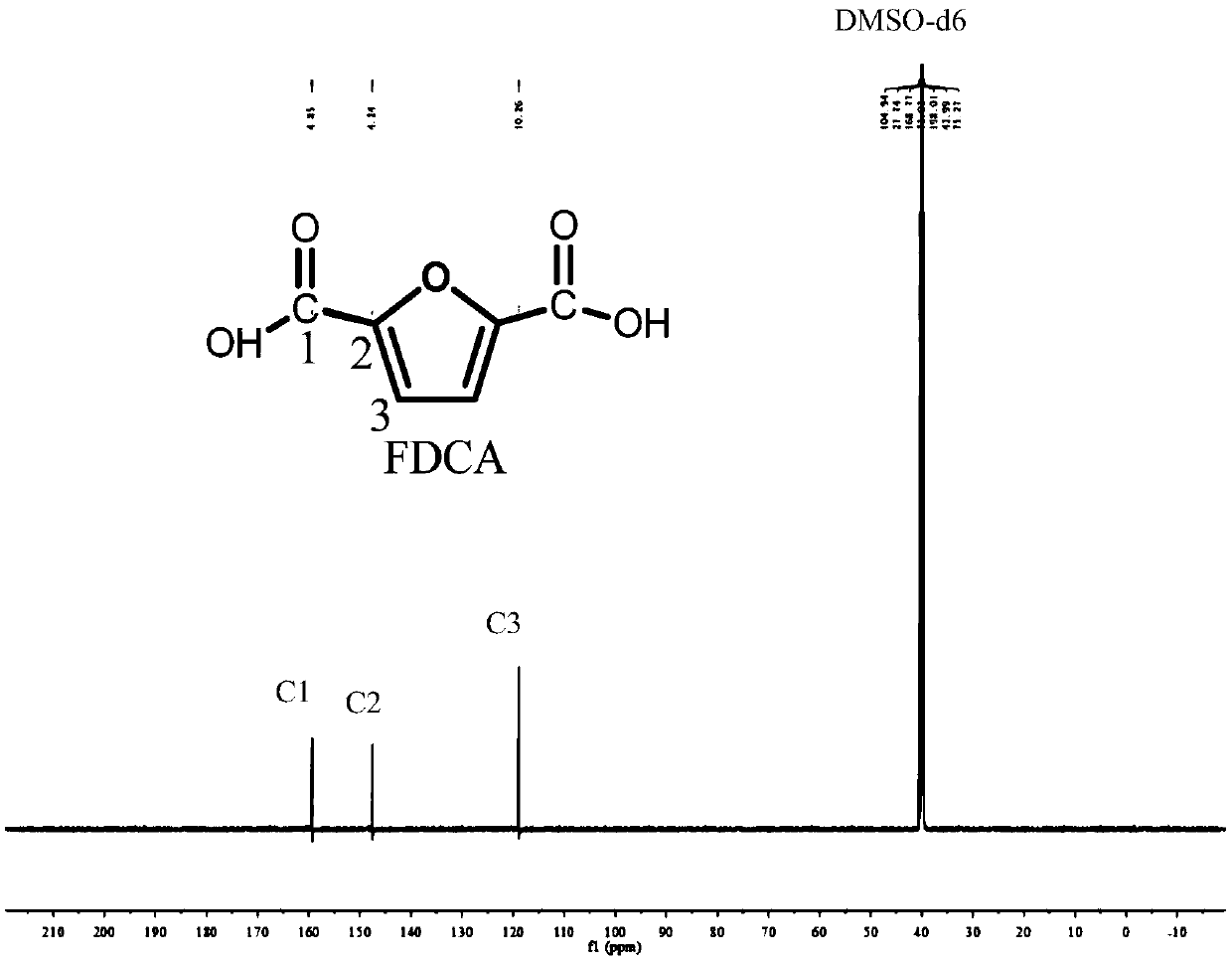
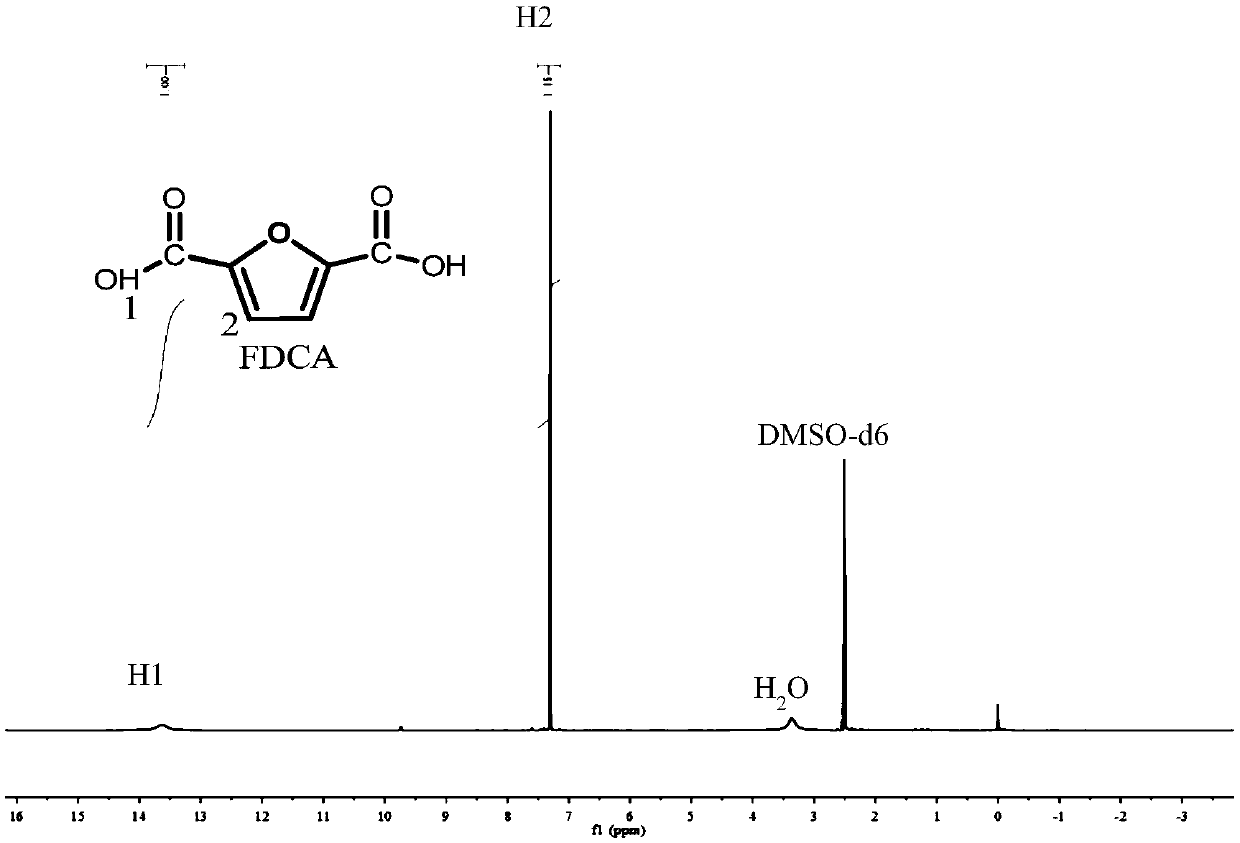
[0025] In the autoclave of 20mL, add 0.7g 5-hydroxymethylfurfural (substrate concentration is 10wt%), 1.57g water and 4.73g polar aprotic solvent, described polar aprotic solvent comprises acetonitrile, dimethyl carbonate , tetrahydrofuran, ethyl acetate, γ-valerolactone, 1,4-epoxyhexane, N,N-dimethylformamide, dimethyl sulfoxide or methyl isobutyl ketone; Add 0.93g of sodium bicarbonate, then add 0.25g of ruthenium carbon (the molar ratio of 5-hydroxymethylfurfural to the active component Ru is 40:1) as a catalyst, add to a sealed reactor, feed 4MPa oxygen, and stir vigorously (500rpm), heated to 110°C and maintained for 10 hours, finished the reaction and cooled to room temperature and took samples, using HPLC (Water 2695) to carry out qualitative and quantitative detection, wherein acetonitrile, dimethyl carbonate, tetrahydrofuran, ethyl acetate, dimethyl The detection results of sulfoxide as a polar aprotic solvent are listed in Table 1 with numbers 1-6.
Embodiment 7~9
[0027] Add 0.7g 5-chloromethylfurfural (10wt% substrate concentration), 1.57g water and 4.73g 1,4-epoxyhexane to a 20mL autoclave, add 0.93g sodium bicarbonate, and then add 0.25g Ruthenium carbon (Ru loading 5%), Ru / ZrO 2 (Ru load 5%), Pt / Al 2 o 3 (Pt load 5%), Pd / ZrO 2 (Pd load 5%), Ru / Co 3 o 4 (Ru load 5%), Pt / Fe 3 o 4 (Pt loading 5%) any one (5-hydroxymethylfurfural and the molar ratio of the active component is 40:1) as a catalyst, add the sealed reactor, feed 4MPa oxygen, vigorously stir (500rpm ), heated to 140°C and maintained for 10 hours, finished the reaction and cooled to room temperature and took samples, using HPLC (Water 2695) for qualitative and quantitative detection, wherein ruthenium carbon (Ru loading 5%), Ru / ZrO 2 (Ru load 5%), Pt / Al 2 o 3 (Pt loading 5%) as the test results of the catalyst are listed in Table 1 as 7-9.
Embodiment 10~14
[0029] In the autoclave of 20mL, add 0.7g 5-hydroxymethyl furfural (substrate concentration is 10wt%), 1.57g water and 4.73g dimethyl sulfoxide, add 0.45g sodium hydroxide, 0.59g sodium carbonate, 0.77g Any one of potassium carbonate, 1.11g potassium bicarbonate or 0.62g potassium hydroxide, then add 0.25g ruthenium carbon (the molar ratio of 5-hydroxymethylfurfural to the active component Ru is 40:1) as a catalyst , add a sealed reactor, feed 4MPa oxygen, stir vigorously (500rpm), heat to 110°C and keep for 10 hours, finish the reaction and cool to room temperature and take samples, use HPLC (Water 2695) to carry out qualitative and quantitative detection, and the detection results are listed in The serial numbers in Table 1 are 10-14.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com