Cyanine near-infrared fluorescent probe as well as preparation method and application thereof
A fluorescent probe, near-infrared technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of crosstalk, small shift of fluorescence emission peak, unfavorable accurate imaging of tumor cells/tissues, etc., and achieve high sensitivity , the effect of simple operation and good industrial application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
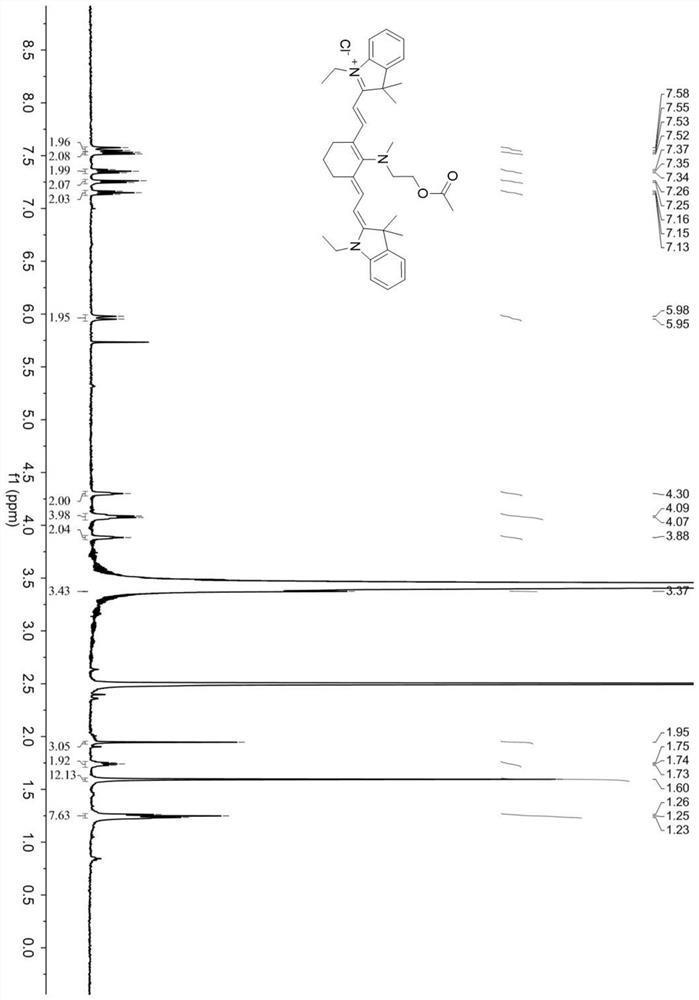
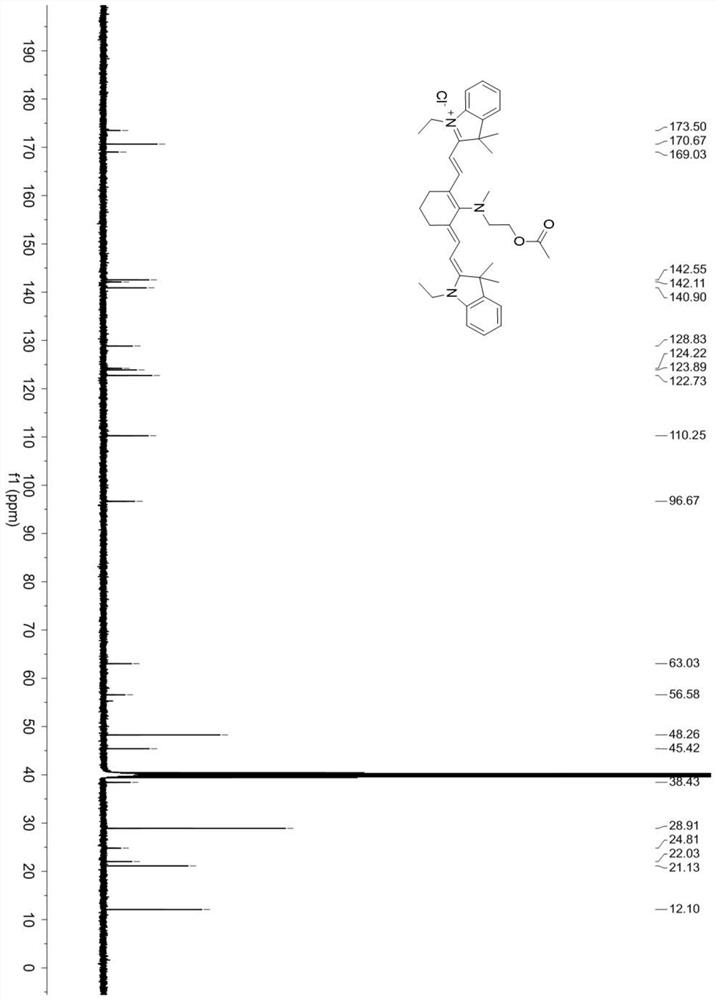
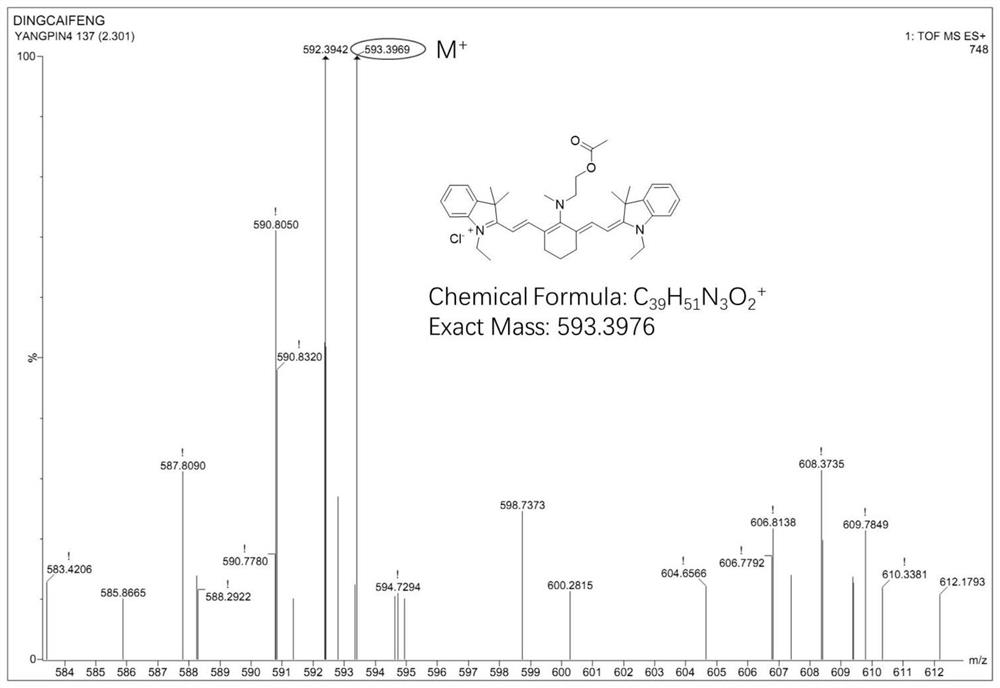
[0054] Synthesis of cyanine near-infrared fluorescent probe compound (Cy-NE):
[0055] Mix CY-N (0.43mmol) and 2.6-lutidine (2.1mmol) in dichloromethane (20mL), heat and stir at 22°C for 10h; after the reaction is over, distill off the solvent under reduced pressure to obtain blue solid; then the crude reaction product was extracted with dichloromethane and water, the solvent was distilled off under reduced pressure, and purified by column chromatography with dichloromethane and methanol to obtain pure fluorescent probe Cy-NE.
Embodiment 2
[0057] Synthesis of cyanine near-infrared fluorescent probe compound (Cy-NE):
[0058] CY-N (0.43mmol) and 2.6-lutidine (3.9mmol) were mixed in dichloromethane (20mL), and acetyl chloride (43.0mmol) diluted with anhydrous dichloromethane was added dropwise under airtight conditions, at room temperature Stir the reaction for 10 hours; after the reaction is completed, the solvent is distilled off under reduced pressure to obtain a blue solid; after the reaction crude product is extracted with dichloromethane and water, the organic phase is distilled off under reduced pressure, and then the solvent is distilled off with dichloromethane and methanol After purification by column chromatography, pure fluorescent probe Cy-NE was obtained.
Embodiment 3
[0060] Synthesis of cyanine near-infrared fluorescent probe compound (Cy-NE):
[0061] CY-N (0.43mmol) and 2.6-lutidine (2.58mmol) were mixed in dichloromethane (20mL), and acetyl chloride (34.0mmol) diluted with anhydrous dichloromethane was added dropwise under airtight conditions, at room temperature Stir the reaction for 10 hours; after the reaction is completed, the solvent is distilled off under reduced pressure to obtain a blue solid; after the reaction crude product is extracted with dichloromethane and water, the organic phase is distilled off under reduced pressure, and then the solvent is distilled off with dichloromethane and methanol After purification by column chromatography, pure fluorescent probe Cy-NE was obtained.
[0062] Each embodiment in this specification is described in a progressive manner, each embodiment focuses on the difference from other embodiments, and the same and similar parts of each embodiment can be referred to each other.
[0063] The co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com