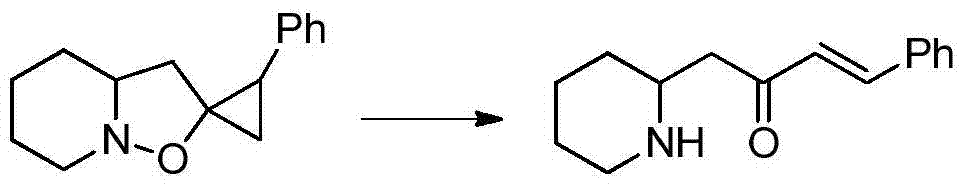
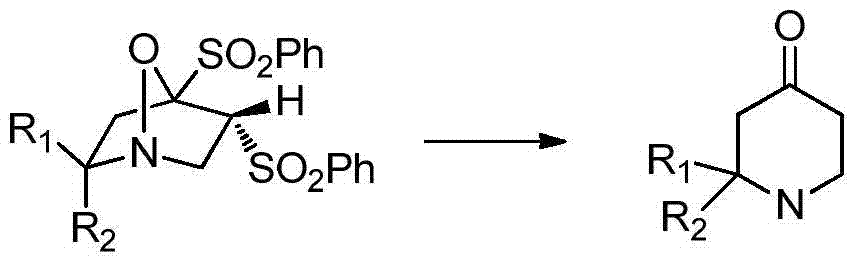
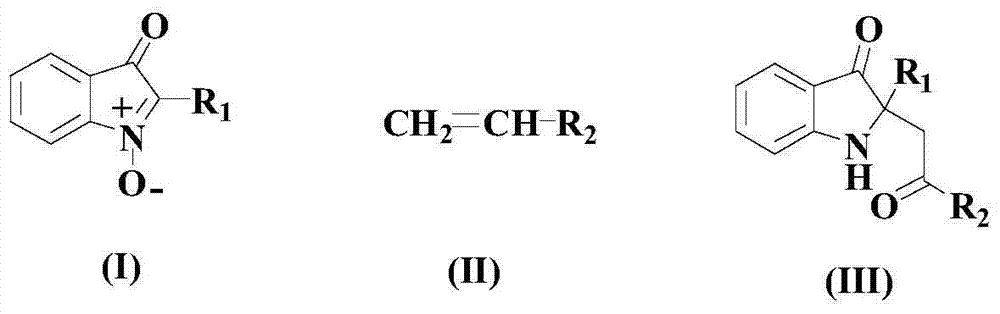
A kind of synthetic method of pharmaceutical intermediate condensed heterocyclic ketone compound
A synthetic method and compound technology, which is applied in the synthesis of ketone compounds, organic synthesis, and the synthesis of fused heterocyclic ketone compounds as pharmaceutical intermediates, achieving the effects of broad market prospects, short process time and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] At room temperature, add 100mmol of the above formula (I) compound, 150mmol of the above formula (II) compound, 6mmol of the catalyst (4mmol Ru 3 (CO) 12 with 2mmol of porphyrin), 30mmol of auxiliary agent (as a mixture of 25mmol of 2-fluorophenylboronic acid pinacol ester and 5mmol of 1,2-bis(triethoxysilyl)ethane) and 100mmol of base DMEDA, and then Heat up to 70°C and fully stir the reaction at this temperature for 10 hours;
[0035] After the reaction is completed, cool the reaction system to room temperature naturally, then adjust the pH value of the system to 6.5-7, then fully shake and wash with deionized water, then add chloroform to extract 2-3 times, combine the organic phases, and dry with anhydrous magnesium sulfate , Distilled under reduced pressure, the residue was chromatographed on a 300-400 mesh silica gel column, with a mixture of ethyl acetate and acetone at a volume ratio of 1:2 as the eluent, TLC detected the elution end point, collect...
Embodiment 2
[0038]
[0039] At room temperature, add 100mmol of the above formula (I) compound, 175mmol of the above formula (II) compound, 7.5mmol of the catalyst (for 5 mmol Ru 3 (CO) 12 with 2.5mmol of porphyrin), 39mmol of auxiliary agent (as a mixture of 26mmol of 2-fluorophenylboronic acid pinacol ester and 13mmol of 1,2-bis(triethoxysilyl)ethane) and 150mmol of base DMEDA, and then Heat up to 75°C and fully stir the reaction at this temperature for 8 hours;
[0040] After the reaction is completed, cool the reaction system to room temperature naturally, then adjust the pH value of the system to 6.5-7, then fully shake and wash with deionized water, then add chloroform to extract 2-3 times, combine the organic phases, and dry with anhydrous magnesium sulfate , Distilled under reduced pressure, the residue was chromatographed on a 300-400 mesh silica gel column, with a mixture of ethyl acetate and acetone at a volume ratio of 1:2 as the eluent, TLC detected the elution end point...
Embodiment 3
[0043]
[0044] At room temperature, add 100mmol of the above formula (I) compound, 200mmol of the above formula (II) compound, 9.9mmol of catalyst (for 6.6 mmol Ru 3 (CO) 12 with 3.3mmol of porphyrin), 50mmol of auxiliary agent (as a mixture of 37.5mmol of 2-fluorophenylboronic acid pinacol ester and 12.5mmol of 1,2-bis(triethoxysilyl)ethane) and 200mmol of base DMEDA , then warming up to 80°C and fully stirring the reaction at this temperature for 6 hours;
[0045] After the reaction is completed, cool the reaction system to room temperature naturally, then adjust the pH value of the system to 6.5-7, then fully shake and wash with deionized water, then add chloroform to extract 2-3 times, combine the organic phases, and dry with anhydrous magnesium sulfate , Distilled under reduced pressure, the residue was chromatographed on a 300-400 mesh silica gel column, with a mixture of ethyl acetate and acetone at a volume ratio of 1:2 as the eluent, TLC detected the elution end...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com