Method for synthesizing 2-acetyl-3-methylpyrazine
A technology of methylpyrazine and acetyl, which is applied in the field of synthesis of flavors and fragrances, can solve the problems of multiple toxic wastes, serious environmental pollution, and application restrictions, and achieve high-efficiency catalytic effects, economical and environmentally friendly synthesis processes, and mild oxidation conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
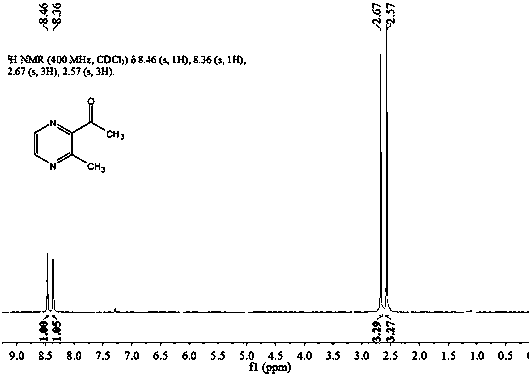
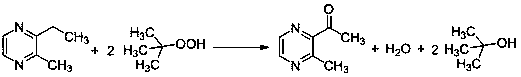
[0021] In a 1000 ml three-necked flask, add 30.0 grams of 2-ethyl-3-methylpyrazine (content 99%), 0.82 grams of ferric chloride (n / n: 50:1), 0.92 grams of 1,10-phenanthrene Roline (n / n: 50:1), 14.8 grams of propionic acid and 145 grams of tert-butanol peroxide were stirred with a magnetic stirrer, heated to an internal temperature of 65-75°C, and the reaction was completed after 24 hours, and then distilled under reduced pressure. After rectification, 2-acetyl-3-methylpyrazine was obtained. The conversion rate of 2-ethyl-3-methylpyrazine was 48.75%, and the selectivity of 2-acetyl-3-methylpyrazine was 95.88%.
Embodiment 2
[0023] In a 1000 ml three-necked flask, add 30.0 g of 2-ethyl-3-methylpyrazine (content 99%), 1.3 g of cobalt (II) acetylacetonate (n / n: 50:1), 0.92 g of 1, 10-Phenanthroline (n / n: 50:1), 14.8 grams of acetic acid and 145 grams of tert-butanol peroxide, stirred with a magnetic stirrer, heated to an internal temperature of 65-75 °C, and the reaction was completed after 24 hours, and the pressure was reduced After distillation and rectification, 2-acetyl-3-methylpyrazine was obtained. The conversion rate of 2-ethyl-3-methylpyrazine was 40.29%, and the selectivity of 2-acetyl-3-methylpyrazine was 90.49%.
Embodiment 3
[0025] In a 1000 ml three-necked flask, add 30.0 g of 2-ethyl-3-methylpyrazine (content 99%), 1.3 g of cobalt (II) acetylacetonate (n / n: 40:1), 0.8 g of 2, 2'-bipyridine (n / n: 40:1), 14.8 grams of benzoic acid and 145 grams of tert-butanol peroxide were stirred with a magnetic stirrer, heated to an internal temperature of 30-40°C, and the reaction was terminated after 24 hours, and the Pressure distillation and rectification give 2-acetyl-3-methylpyrazine. The conversion rate of 2-ethyl-3-methylpyrazine was 37.05%, and the selectivity of 2-acetyl-3-methylpyrazine was 92.23%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com