Polyalliin and preparation method thereof
A technology of polyalliin and polydeoxyalliin, which is applied in the field of polymers, can solve the problem that the product structure depends on oxidation conditions, and achieve good biocompatibility, high efficiency, and mild oxidation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
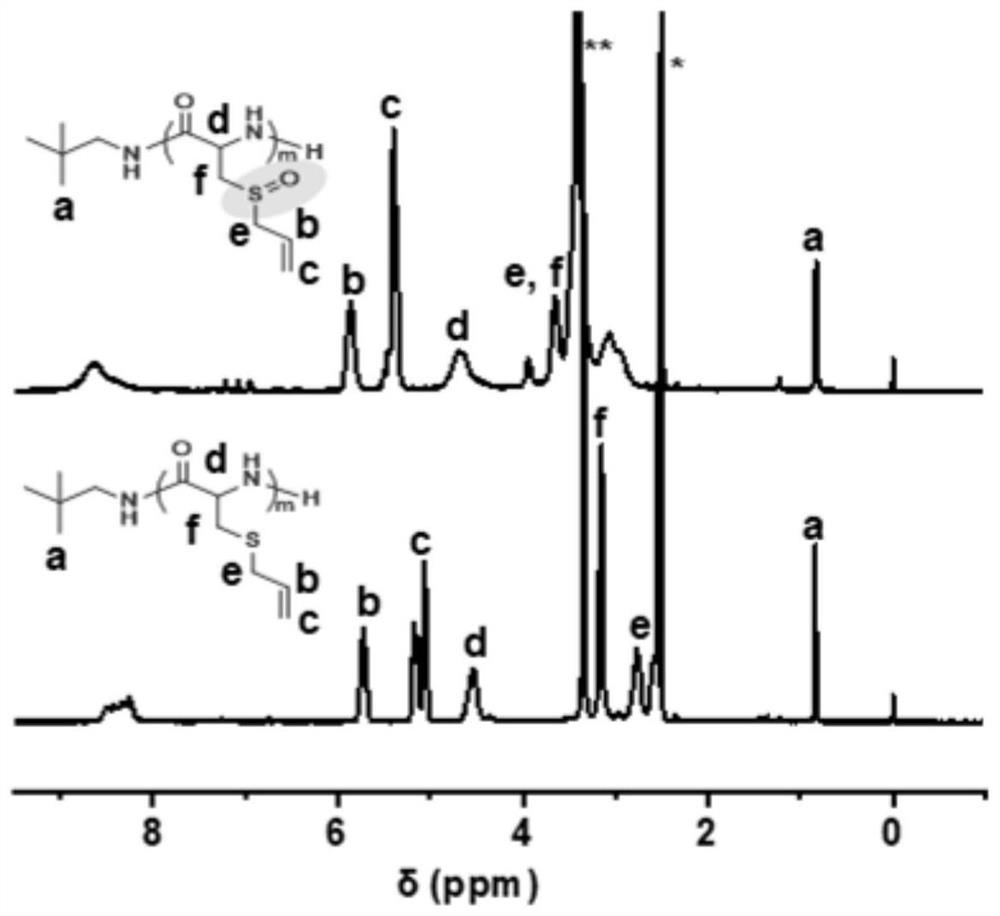
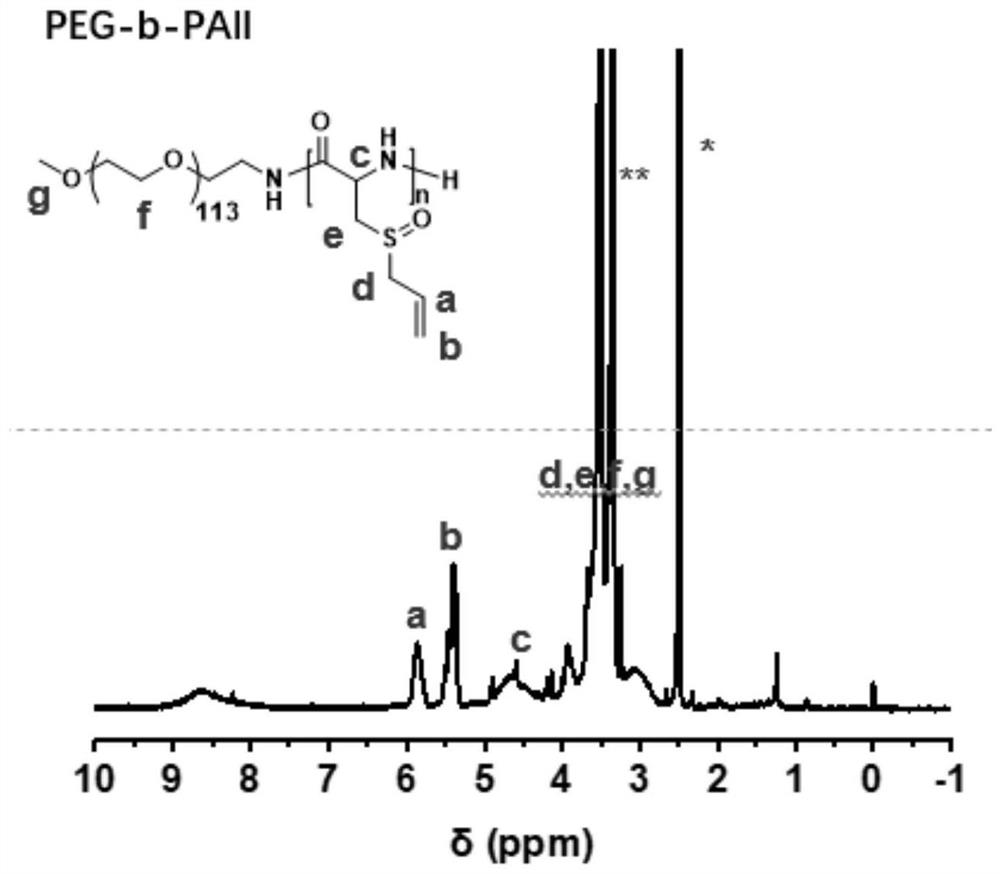
[0044] (1) 2.8130g (10.0mmol) of deoxyalliin-NPC was added to the reaction flask, dissolved with 7.4mL DMSO, and then the DMSO solution (0.1270mol / L, 0.3303mmol) of 2.6mL neopentylamine was added, and the The molar ratio of amino acid-NPC monomer to neopentylamine was 30:1, sealed and placed in an oil bath at 80 °C for 2 days. After the reaction, the original solution was directly poured into ether for precipitation, centrifugation, and the supernatant was discarded. , the obtained polymer was vacuum-dried for 1 day to obtain neopentylamine-terminated polydeoxyalliin with a yield of 71% and a number-average molecular weight of 3.7kDa.
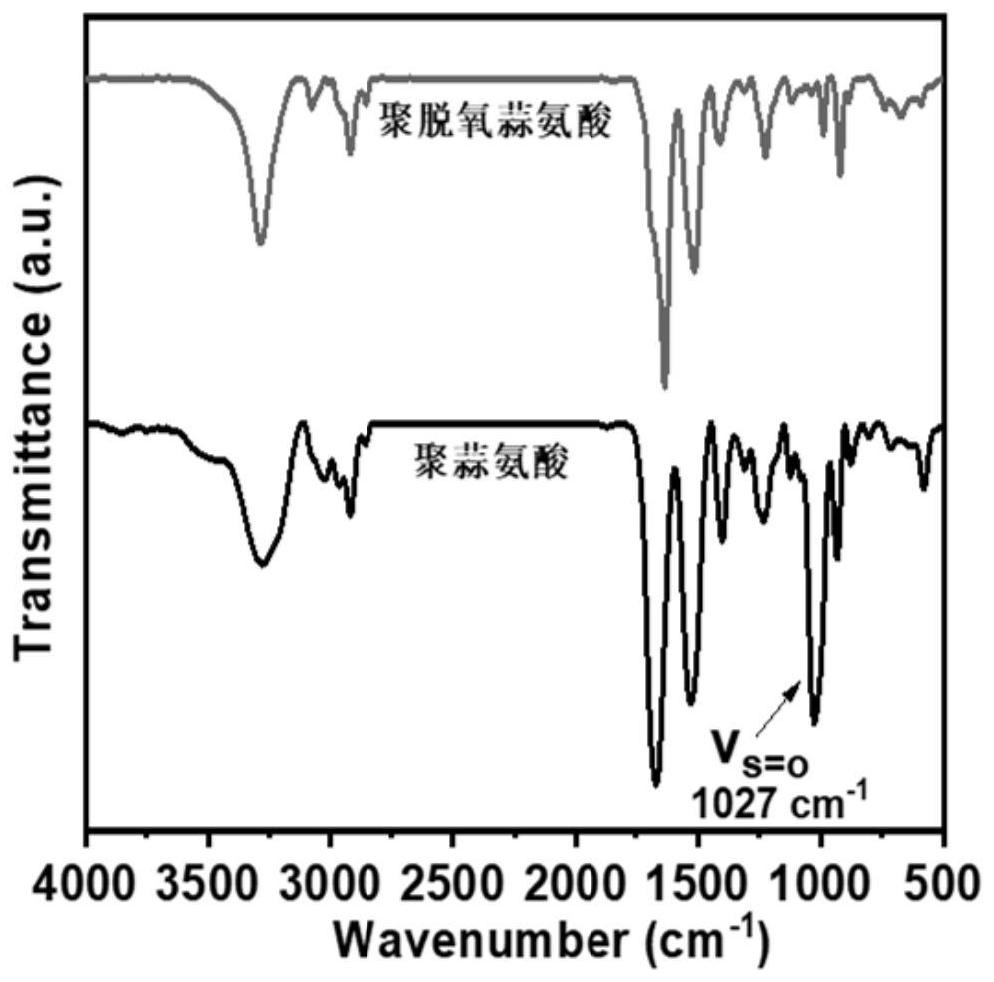
[0045] (2) 0.0603 g of polydeoxyalliin (end-capped with neopentylamine, number-average molecular weight of 3.7kDa) (containing 0.4195 mmol of repeating units of deoxyalliin), dissolved in 6 mL of hexafluoroisopropanol, and added with 85 μL (30 wt% ) of H 2 O 2 (0.8485mmol), H 2 O 2The molar ratio with the repeating unit of polydeoxyalliin i...
Embodiment 2
[0047] Polydeoxyalliin (n-hexylamine-terminated) was prepared according to step (1) of Example 1, except that neopentylamine was replaced with n-hexylamine.
[0048] Polydeoxyalliin (n-hexylamine end-capped, number-average molecular weight is 4.8kDa) is a raw material, and other oxidation conditions are the same as in Example 1, except that H 2 O 2 The molar ratio with the repeating unit of polydeoxyalliin is 5:1, and the reaction time is 3h. The yield of the obtained product polyaliin was 96%.
Embodiment 3
[0050] Other conditions are the same as in Example 1, the difference is that the number-average molecular weight of polydeoxyalliin is 2.5kDa, and the H 2 O 2 The ratio of the repeating unit to polydeoxyalliin is 8:1, the reaction temperature is 10° C., the reaction time is 3h, and the yield of the obtained product polyaliin is 94%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com