Method for producing cyclohexylamine by hydrogenating aniline
A technology of cyclohexylamine and aniline, which is applied in the field of hydrogenation of aniline to produce cyclohexylamine, can solve the problems of low yield and selectivity of cyclohexylamine, and achieve the effect of improving yield and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
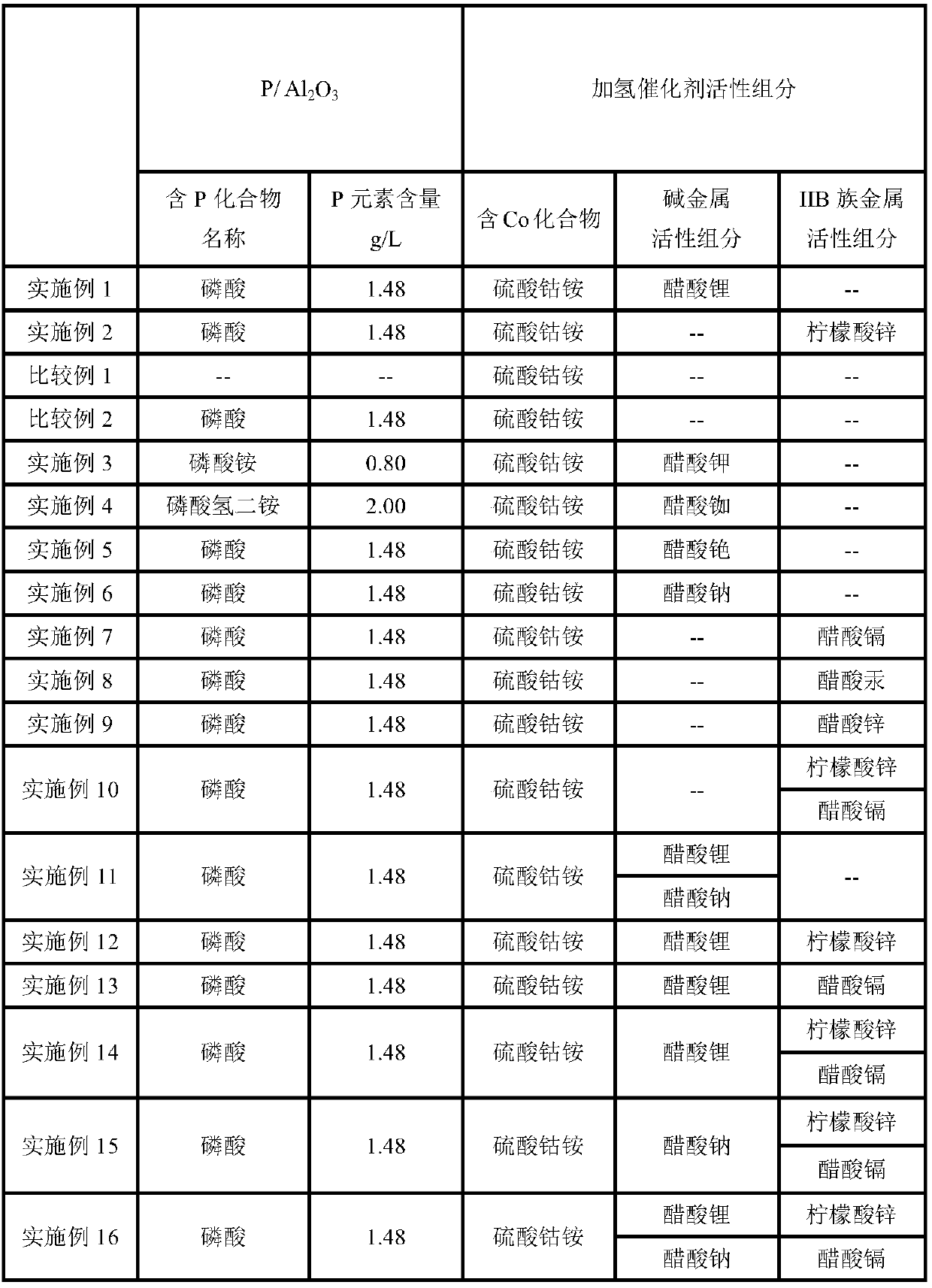
Embodiment 1
[0044] Modified carrier P / Al 2 o 3 Preparation of:
[0045] (1) Phosphoric acid (H 3 PO 4 ) aqueous solution 180ml impregnated in 1L diameter 6mm, pore volume is 0.92cm 3 / g, the specific surface area is 200cm 2 / g Al 2 o 3 , standing for 24 hours, and drying at 110° C. for 4 hours to obtain the carrier precursor I.
[0046] (2) Calcining the carrier precursor I at 630°C for 5h under a nitrogen gas atmosphere to obtain the modified carrier P / Al 2 o 3 .
[0047] The P content in the carrier was determined to be 1.48 g / L by ICP.
[0048] Preparation of hydrogenation catalyst:
[0049] (i) Cobalt ammonium sulfate containing 2.18g Co ((NH 4 ) 2 Co(SO 4 ) 2 ·6H 2 O) and 200ml of aqueous solution of lithium acetate (LiOAc) containing 1.88g Li is impregnated in P / Al 2 o 3 , to obtain catalyst precursor I;
[0050] (ii) drying at 110° C. for 4 hours to obtain the catalyst.
[0051] The Co content of the catalyst measured by ICP is 2.18g / L, and the Li content is 1.8...
Embodiment 2
[0056] Modified carrier P / Al 2 o 3 Preparation of:
[0057] (1) Phosphoric acid (H 3 PO 4 ) aqueous solution 180ml impregnated in 1L diameter 6mm, pore volume is 0.92cm 3 / g, the specific surface area is 200cm 2 / g Al 2 o 3 , standing for 24 hours, and drying at 110° C. for 4 hours to obtain the carrier precursor I.
[0058] (2) Calcining the carrier precursor I at 630°C for 5h under a nitrogen gas atmosphere to obtain the modified carrier P / Al 2 o 3 .
[0059] The P content in the carrier was determined to be 1.48 g / L by ICP.
[0060] Preparation of hydrogenation catalyst:
[0061] (i) Cobalt ammonium sulfate containing 2.18g Co ((NH 4 ) 2 Co(SO 4 ) 2 ·6H 2 O) and zinc citrate (Zn 3 (C 6 h 5 o 7 ) 2 2H 2 O) 200ml of aqueous solution impregnated in P / Al 2 o 3 , to obtain catalyst precursor I;
[0062] (ii) drying at 110° C. for 4 hours to obtain the catalyst.
[0063] The Co content of the catalyst was measured by ICP to be 2.18g / L, and the Zn content...
Embodiment 3
[0092] Modified carrier P / Al 2 o 3 Preparation of:
[0093] (1) Ammonium phosphate (NH 4 ) 3 PO 4 ) aqueous solution 180ml impregnated in 1L diameter 6mm, pore volume is 0.92cm 3 / g, the specific surface area is 200cm 2 / g Al 2 o 3 , standing for 24 hours, and drying at 110° C. for 4 hours to obtain the carrier precursor I.
[0094] (2) Calcining the carrier precursor I at 630°C for 5h under a nitrogen gas atmosphere to obtain the modified carrier P / Al 2 o 3 .
[0095] The P content in the carrier was determined by ICP to be 0.80 g / L.
[0096] Preparation of hydrogenation catalyst:
[0097] (i) Cobalt ammonium sulfate containing 2.18g Co ((NH 4 ) 2 Co(SO 4 ) 2 ·6H 2 O) and 200ml of aqueous solution of potassium acetate (KOAc) containing 1.88g K is impregnated in P / Al 2 o 3 , to obtain catalyst precursor I;
[0098] (ii) drying at 110° C. for 4 hours to obtain the catalyst.
[0099] The Co content of the catalyst was measured by ICP to be 2.18g / L, and the K...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com