Novel broad-spectrum antimicrobial peptide SAMP1-A4 and preparing method thereof
An antibacterial peptide, broad-spectrum technology, applied in the field of novel antibacterial peptides and their preparation, can solve the problems of unstable expression product, low product yield, easy to be degraded, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of Antimicrobial Peptide SAMP1-A4
[0019] The target peptide was synthesized by the solid-phase Fmoc method from the C-terminus to the N-terminus. The carboxyl group of the first Fmoc-Ile-OH at the C-terminus of the polypeptide is activated and combined with the resin, the Fmoc-protecting group is removed, and the Fmoc-Arg(Pbf)-OH activated by the second carboxyl group is combined, and the Fmoc-Ile-OH is removed. Protecting group, the cycle is repeated until all amino acids are added. Cut the peptide on the resin with cutting solution, collect the cutting solution, dry it in a centrifuge tube, wash the precipitate with ice ether 2~3 times, centrifuge to remove the supernatant, and dry the precipitate. The obtained crude product was purified by HPLC.
Embodiment 2
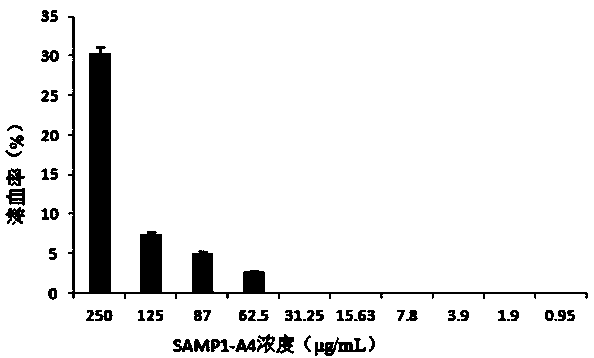
[0020] Example 2 Antimicrobial Spectrum Determination of Antimicrobial Peptide SAMP1-A4
[0021] 1) Weigh and dissolve protein. Prepare the SAMP1-A4 phosphoric acid solution, set the initial concentration in the first column to 500 μg / mL, and set the final concentration to 0.975 μg / mL. It is necessary to prepare 2mg / mL SAMP1-A4 stock solution. Dissolve SAMP1-A4 with 20 mmol / L sodium phosphate buffer solution pH6.0, and filter the solution through a 0.22 μm sterile filter membrane to sterilize.
[0022] 2) Using a pipette gun, add 100 μL of 20 mmol / L pH6.0 sodium phosphate buffer to each well of the 96-well plate to dilute SAMP1-A4.
[0023] 3) Use a pipette gun to pipette 100 μL of 2 mg / mL SAMP1-A4 stock solution into each well of the first column of the 96-well plate.
[0024] 4) Repeatedly blow and aspirate the solution in the first column of the plate for 6-8 times, mix well without splashing.
[0025] 5) Aspirate 100 μL from the first column and add it to the second co...
Embodiment 3
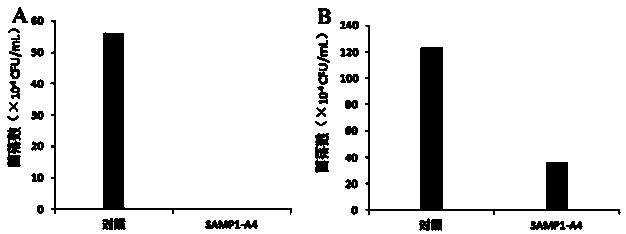
[0037] Example 3 Determination of antibacterial peptide SAMP1-A4 bactericidal and antibacterial activity
[0038] The bactericidal and antibacterial activity of SAMP1-A4 was detected by measuring the number of viable bacteria / fungi after the action of antimicrobial peptides. Candida tropicalis is the representative fungus, and Listeria monocytogenes is the representative bacterial strain.
[0039] Candida tropicalis: culture Candida tropicalis at 28°C with SD liquid medium to OD 600 ≈0.6-0.8. Add the bacterial suspension into the solution containing 1×MIC 100 In the SD liquid medium of SAMP1-A4, the bacterial concentration was about 1×10 5 CFU / mL. At the same time, a blank SD medium without SAMP1-A4 was set as a control. Cultivate at a constant temperature in a shaker at 28°C. After culturing for 4 hours, absorb 100 μL of the bacterial solution, spread it on an SD agar plate after dilution, and culture overnight at 28°C for colony counting.
[0040] Listeria monocytoge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com