Method for preparing nonoxynol without ethylene oxide
A technology of ethylene oxide and nonoxynol, which is applied in the field of medicine and chemical industry, can solve the problems of ethylene oxide residue in nonoxynol, reduce the content of ethylene oxide, be simple to operate, and facilitate industrial scale-up production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
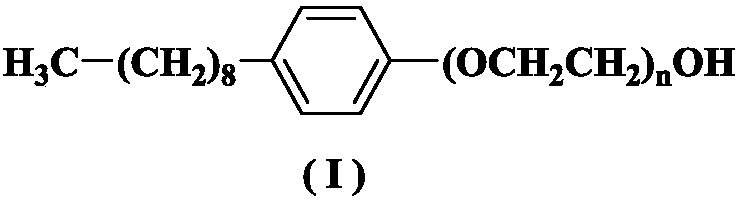
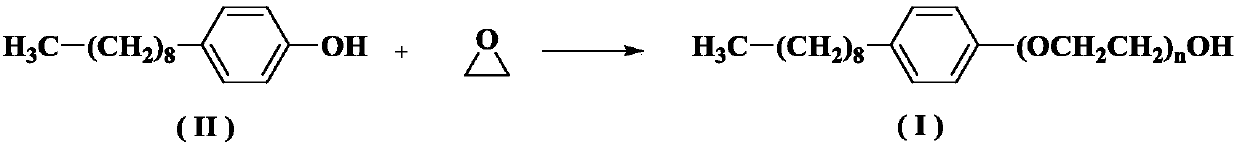
[0039] Add nonylphenol (100g, 0.45mol) and sodium hydroxide (0.25g, 6.25mmol) into the reaction kettle, and replace the air with nitrogen; raise the temperature to 140-160°C. Control the temperature at 140-160°C (keep the temperature at 140-160°C, keep this temperature when feeding ethylene oxide later and during the reaction, the same below), feed ethylene oxide (200g, 4.54mol), Ethylene oxide passing time is about 4 hours. After the introduction, take a sample to detect the cloud point (should be 52-56°C). After the cloud point is qualified, add sodium hydroxide (0.5g, 12.5mmol) and continue the reaction for 0.5h. Stop the reaction, lower the temperature to 65-75°C, and wash with saturated aqueous sodium chloride solution 2-3 times. Vacuum drying at 100°C for 2 hours gave 290 g of a colorless oil. Residual amount of ethylene oxide: not detected.
Embodiment 2
[0041] Add nonylphenol (100g, 0.45mol) and sodium hydroxide (0.25g, 6.25mmol) into the reaction kettle, and replace the air with nitrogen; raise the temperature to 140-160°C. The temperature was controlled at 140-160°C, and ethylene oxide (200 g, 4.54 mol) was passed in for about 4 hours. After the introduction, take a sample to detect the cloud point (should be 52-56°C). After the cloud point is qualified, add sodium hydroxide (0.75g, 18.75mmol) and continue the reaction for 0.5h. Stop the reaction, lower the temperature to 65-75°C, and wash with saturated aqueous sodium chloride solution 2-3 times. Vacuum drying at 100°C for 2 hours gave 288 g of light yellow oil. Residual amount of ethylene oxide: not detected.
Embodiment 3
[0043] Add nonylphenol (100g, 0.45mol) and sodium hydroxide (0.25g, 6.25mmol) into the reaction kettle, and replace the air with nitrogen; raise the temperature to 160-180°C. The temperature is controlled at 160-180°C, and ethylene oxide (200 g, 4.54 mol) is introduced, and the time for introducing ethylene oxide is about 4 hours. After the introduction, take a sample to detect the cloud point (should be 52-56°C). After the cloud point is qualified, add sodium hydroxide (0.75g, 18.75mmol) and continue the reaction for 0.5h. Stop the reaction, lower the temperature to 65-75°C, and wash with saturated aqueous sodium chloride solution 2-3 times. Vacuum drying at 100°C for 2 hours gave 280 g of light yellow oil. Residual amount of ethylene oxide: not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com