Preparation method for metal coordinated polymer long-life luminescent material and application
A compound and metal salt technology, which is applied in the field of metal coordination polymer long-life luminescent materials and its preparation, can solve the problems of high price, environmental pollution, and limited storage of rare heavy metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Synthesis of Example 1 Compound L1 (5,10-bis(pyridin-4-yl)-5,10-dihydrophenazine)
[0113]
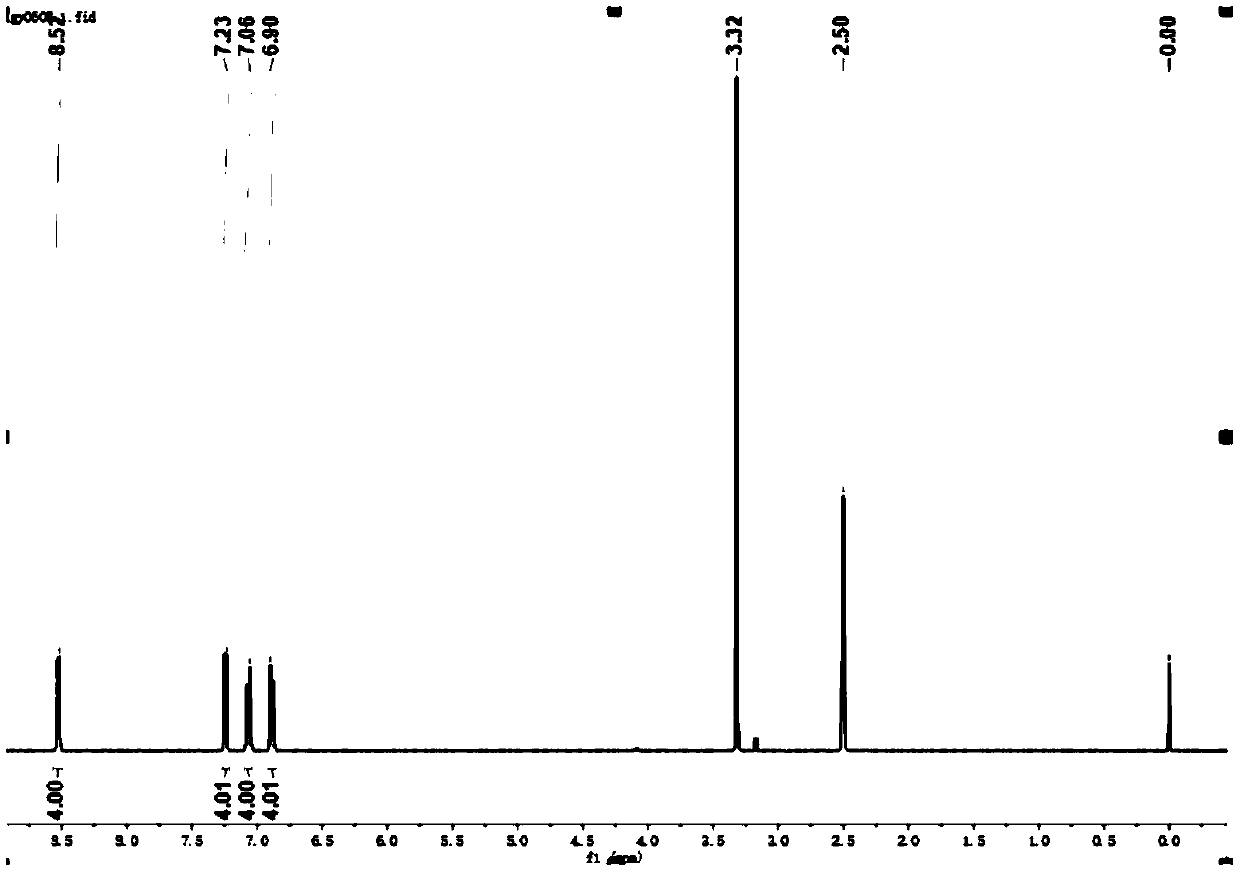
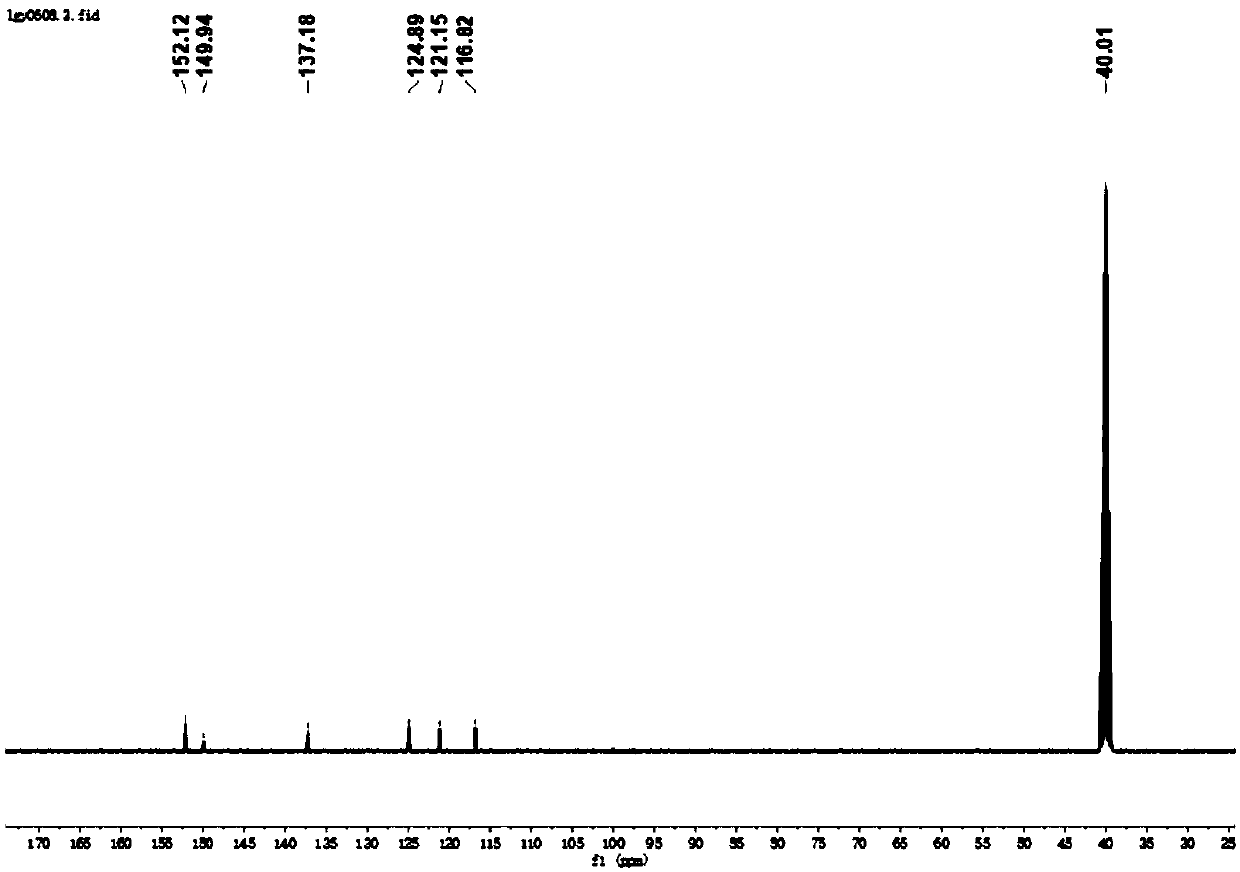
[0114] Under nitrogen protection, 5,10-dihydrophenazine (0.92g, 5mmol), 4-bromopyridine (1.9g, 12mmol), Pd(OAc) 2 (68mg, 0.3mmol), t-BuONa (3.46g, 36mmol), [(t-Bu) 3 PH]BF 4 (262mg, 0.9mmol) and toluene (50mL) were stirred at 110°C for 16 hours. After cooling to room temperature, water (100 mL) was added. layered. The aqueous phase was extracted with DCM (3 x 50 mL), the combined organic phases were dried over anhydrous sodium sulfate, filtered and the solvent was removed. Using dichloromethane as eluent, pass Al 2 o 3 The residue was purified by column chromatography to obtain the product L1 as a green solid (0.82 g, 48.7%), and its hydrogen and carbon spectra were as follows: figure 1 , 2 As shown, the CIE diagram is shown as Figure 12 As shown, the fluorescence emission pattern of PL (photoluminescence) is shown as Figure 13 shown.
[0115] 1 H NMR (400MHz,...
Embodiment 2
[0116] Synthesis of Example 2 Compound L2 (10,10'-bis(pyridin-3-yl)-10H,10'H-9,9'-spiroacridine)
[0117]
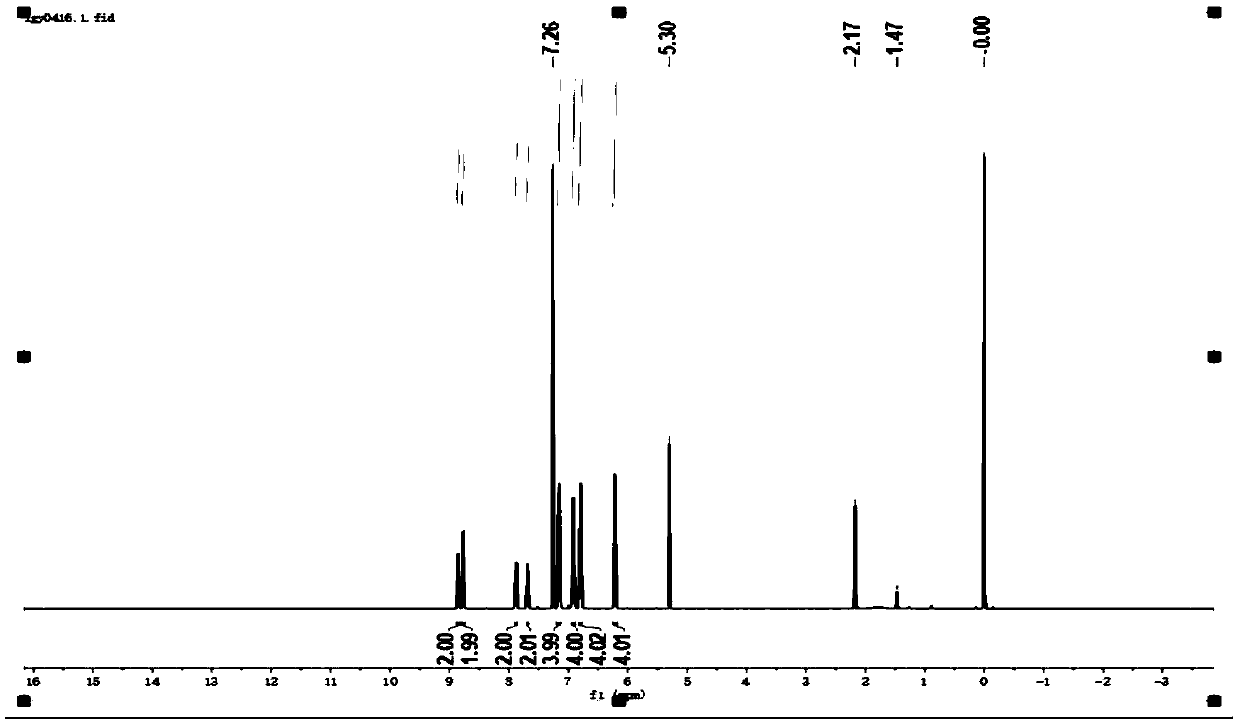
[0118] Under nitrogen protection, 10H, 10'H-9,9'-spiroacridine (1.04g, 3mmol), 3-bromopyridine (1.26g, 8mmol), Pd(OAc) 2 (68mg, 0.3mmol) mixture, add t-BuONa (3.46g, 36mmol), [(t-Bu) 3 PH]BF 4 (262mg, 0.9mmol) and toluene (50mL) were stirred at 110°C for 16 hours. After cooling to room temperature, water (100 mL) was added, the layers were separated, the aqueous phase was extracted with DCM (3 x 50 mL), the combined organic phases were dried over sodium sulfate, filtered and the solvent was removed. The residue was purified by silica gel column chromatography, using dichloromethane: acetone (v / v=10:1) as eluent to obtain white solid product L2 (1.06g, 70.6%), and its hydrogen and carbon spectra were as follows: image 3 , 4 As shown, the CIE diagram is shown as Figure 12 As shown, the fluorescence emission pattern of PL (photoluminescence) is shown as Figure...
Embodiment 3
[0120] Example 3 Compound R-1 (i.e. 4,4'-(10H,10'H-9,9'-spirobis[acridine]-10,10'-diyl)diphenylcarbaline Acid methyl ester) synthesis
[0121]
[0122] Under nitrogen protection, 10H, 10H'-9,9-spiroacridine (1mmol, 0.34g), methyl 4-bromobenzoate (3mmol, 0.65g), cesium carbonate (5mmol, 1.64g), palladium acetate ( 0.13mmol, 30mg) was placed in a 100mL three-necked bottle, 50mL of anhydrous toluene was added, and 1.2mL (0.41mmol) (tBu) was injected into the syringe 3 P, stirred at 110°C for 24h. After cooling to room temperature, the whole system was poured into 100 mL of water, and the layers were separated. Extract with dichloromethane (3 x 50 mL), combine the organic phases, dry over anhydrous magnesium sulfate, and filter. The solvent was removed under reduced pressure, and silica gel column chromatography (CH 2 Cl 2 ) to obtain white solid 0.4g, productive rate is 65%, and its hydrogen spectrum, carbon spectrum are as Figure 5 , 6 shown.
[0123] 1 H NMR (400M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com