Nimesulide derivatives and preparation method and application thereof
A nimesulide and derivative technology, applied in the field of preparation of nimesulide derivatives, can solve the problems of mitochondrial permeability change, low nimesulide solubility, mitochondrial failure, etc., and achieve strong feasibility and repeatability Sexuality, cost reduction, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
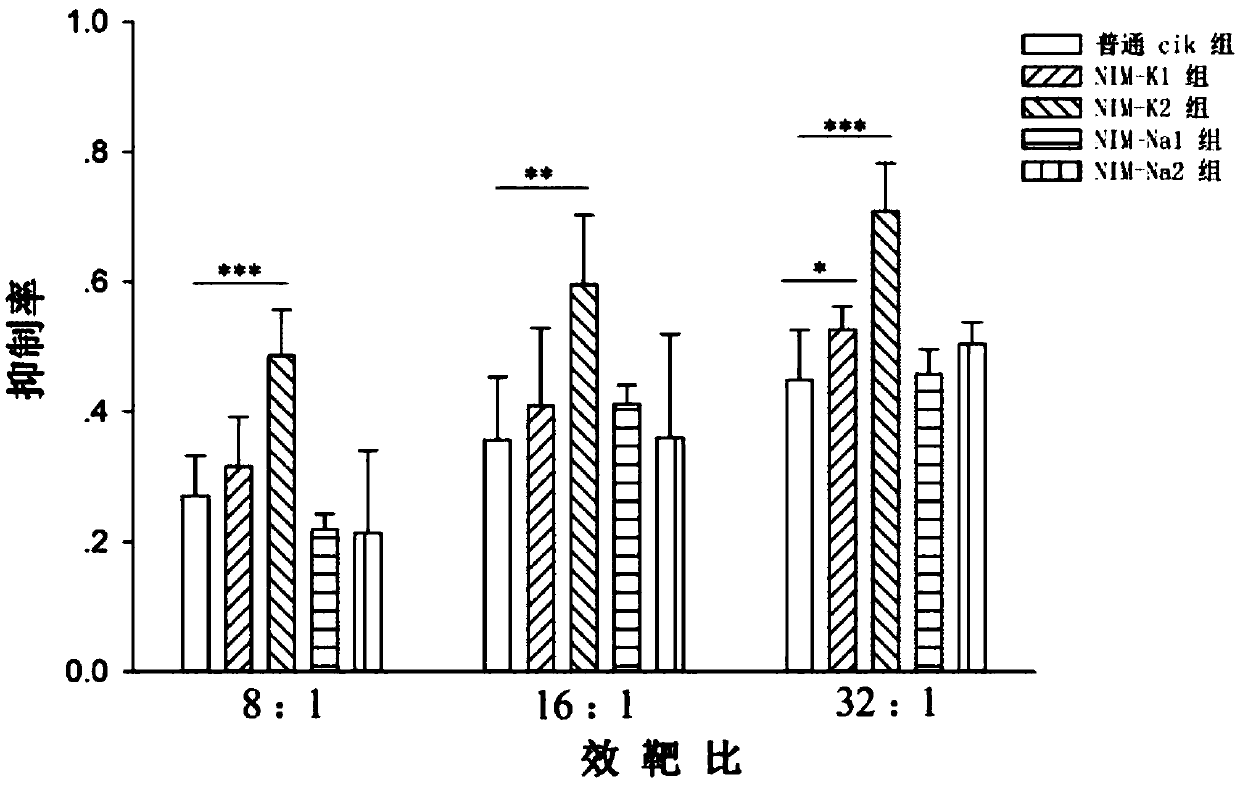
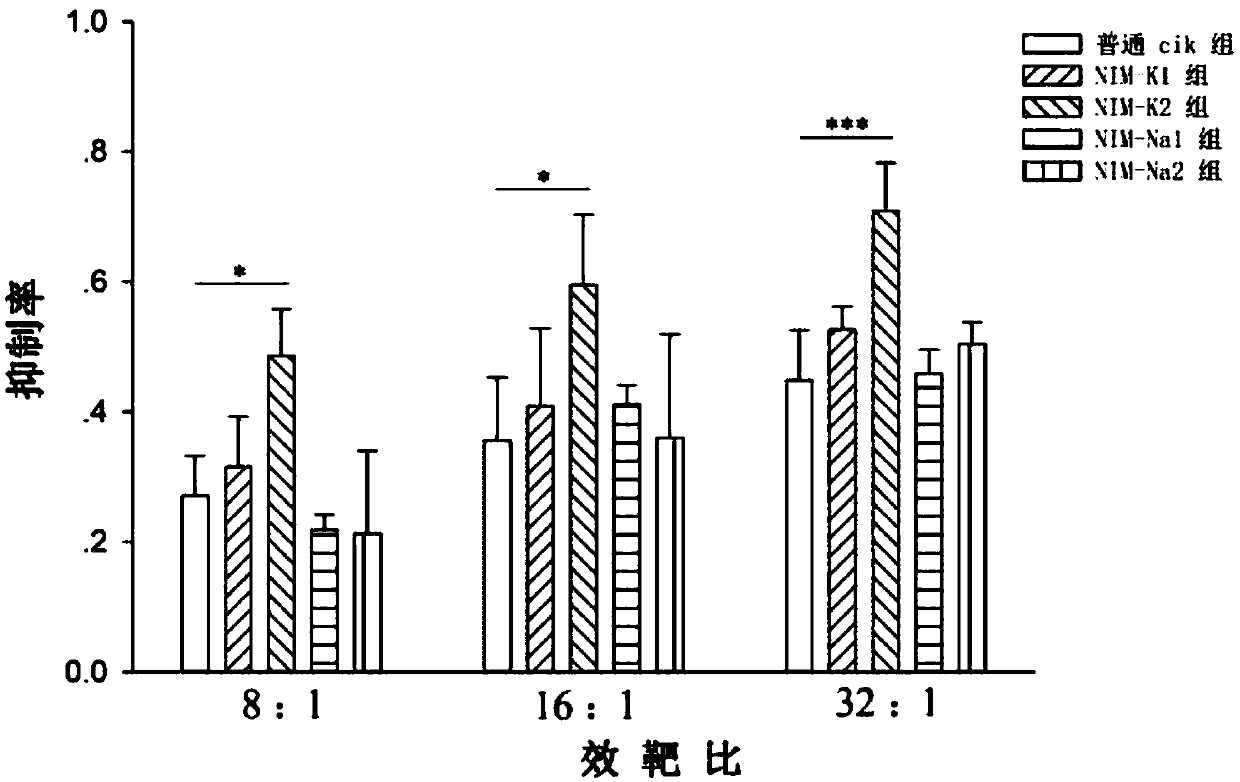
Image
Examples
Embodiment 1
[0073] The molecular formula of the nimesulide derivative in the present embodiment is: C 13 h 11 N 2 NaO 5 S;
[0074] The chemical structural formula is:
[0075]
[0076] Molecular weight: 330.03.
[0077]The synthetic route of the nimesulide derivative described in this example is:
[0078]
[0079] The preparation steps of the nimesulide derivative are:
[0080] Dissolve nimesulide in distilled water to make nimesulide aqueous solution, the concentration of nimesulide aqueous solution is 0.4g / ml, add the aqueous solution of sodium hydroxide to the nimesulide aqueous solution, the concentration of sodium hydroxide aqueous solution is 0.1g / ml, the molar ratio of nimesulide to sodium hydroxide added was 0.8:1, the system was heated up to 45°C, the insoluble matter was completely dissolved, and the stirring was continued for 1h, and after cooling to room temperature, it was transferred to an ice bath at 0°C. A yellow solid was precipitated, and the solid was was...
Embodiment 2
[0082] The molecular formula of the present embodiment nimesulide derivative is: C 13 h 11 N 2 KO 5 S;
[0083] The chemical structural formula is:
[0084]
[0085] Molecular weight: 346.00.
[0086] The synthetic route of the nimesulide derivative described in this example is:
[0087]
[0088] The preparation steps of the nimesulide derivative are:
[0089] Dissolve nimesulide in distilled water to make nimesulide aqueous solution, the concentration of nimesulide aqueous solution is 0.4g / ml, add the aqueous solution of potassium hydroxide to the nimesulide aqueous solution, the concentration of potassium hydroxide aqueous solution is 0.1g / ml, the molar ratio of nimesulide to potassium hydroxide added is 0.8:1, the system is heated up to 45°C, the insoluble matter is completely dissolved, and the stirring is continued for 1h, cooled to room temperature and then transferred to an ice bath at 0°C, a large amount of A yellow solid was precipitated, and the solid w...
Embodiment 3
[0091] The molecular formula of the present embodiment nimesulide derivative is: C 13 h 14 N 2 o 3 S;
[0092] The chemical structural formula is:
[0093]
[0094] Molecular weight: 278.07.
[0095] The synthetic route of the nimesulide derivative described in this example is:
[0096]
[0097] The preparation steps of the nimesulide derivative are:
[0098] Mix nimesulide, tin and concentrated hydrochloric acid, the concentration of concentrated hydrochloric acid is 12mol / L, the molar ratio of nimesulide, tin and hydrochloric acid is 6:11:1, reflux at 85°C for 5h, pour it after the reaction is complete into ice water, filter out the solid and basify it with lye, and then recrystallize with a mixed solvent of methanol and chloroform to obtain a light brown solid that is a nimesulide derivative.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com