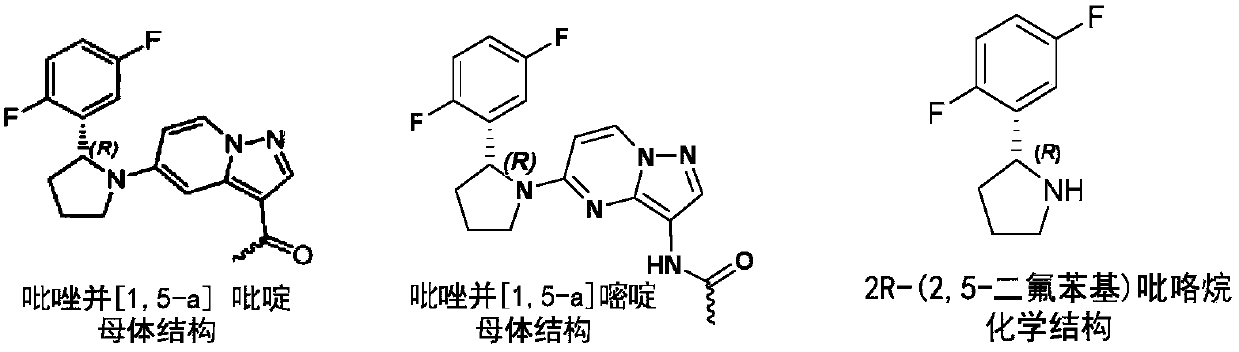
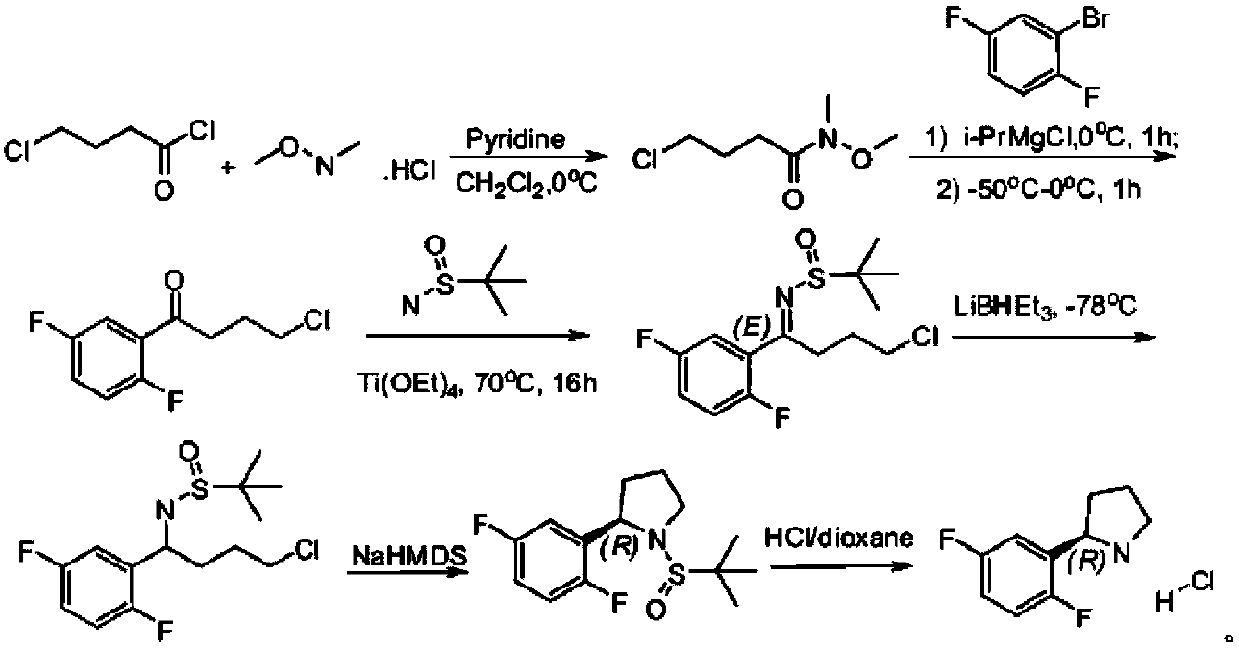
Preparation method of (R)-2-(2,5-difluorophenyl)pyrrolidine or salt thereof
A kind of technology of difluorophenyl, pyrrolidine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 recombinant imine reductase
[0038] Inoculate a single colony of recombinant Escherichia coli containing the imine reductase gene from a glycerol tube or transformation plate into 4 mL of liquid LB medium containing (100 ug / mL) ampicillin resistance, and activate overnight at 37 ° C for 12 to 16 hours. The culture obtained after activation was transferred to 100mL liquid LB medium containing (100ug / mL) ampicillin resistance with 2% inoculum, and cultured with shaking at 37°C and 220rpm until the OD600 value reached about 0.6, and the inducer isotropic acid was added. Propyl-β-D-thiogalactoside was added to a final concentration of 0.8 mmol / L, and culture was continued overnight at 30°C. Collect the cells by centrifugation (4°C, 5000 rpm, 15 min), and suspend the cells with 10 mL of phosphate buffer (100 mM, pH 7.0). Place the cell suspension in an ice bath for 10 minutes, then centrifuge (4°C, 12000rpm, 15min), pre-freeze the supernatan...
Embodiment 2
[0039] Example 2 Synthesis of (R)-2-(2,5-difluorophenyl)pyrrolidine (compound 5 free base) 1 tert-butyl (4-(2,5-difluorophenyl) 4-carbonyl Synthesis of butyl) carbamate (compound 3)
[0040]
[0041] In a 250mL flask, under nitrogen protection, add N-Boc protected pyrrolidone (18.5g, 0.1mol), 50mL tetrahydrofuran, cool to below 25°C, add dropwise 2,5-difluorophenylmagnesium chloride lithium Grignard reagent ( 120mL, 0.12mol, 1M tetrahydrofuran solution, obtained by Grignard exchange of diisopropylmagnesium lithium chloride), after the dropwise addition was completed, the temperature was raised to 25°C, and the temperature was kept stirring for 5 hours, and the reaction was detected by TLC. Dilute hydrochloric acid was added dropwise to quench the reaction solution to pH 6-7, the mixture was extracted three times with dichloromethane, 60 mL each time, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a cr...
Embodiment 3
[0058] Example 3 Synthesis steps of (R)-2-(2,5-difluorophenyl)pyrrolidine (compound 5 bisulfate) 1 tert-butyl (4-(2,5-difluorophenyl) 4- Synthesis of Carbobutyl) Carbamate (Compound 3)
[0059]
[0060] In a 250mL flask, under nitrogen protection, add N-Boc-protected pyrrolidone (18.5g, 0.1mol), 2-methyltetrahydrofuran (40mL), cool to below 25°C, add dropwise 2,5-difluorophenylmagnesium chloride Lithium chloride Grignard reagent (140mL, 0.14mol, 1M 2-methyltetrahydrofuran solution, obtained by Grignard exchange of diisopropylmagnesium lithium chloride), after the dropwise addition was completed, the temperature was raised to 30°C, and the temperature was kept stirring for 4 hours. TLC detects that the reaction is complete. Add dilute sulfuric acid dropwise to quench the reaction solution to pH 6-7, the mixture is separated, the water layer is extracted twice with 2-methyltetrahydrofuran, 50 mL each time, the organic phase is combined, the temperature is lowered to below 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com