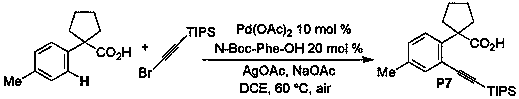
Phenylacetic acid type aryne preparation method
A technology of phenylacetic acid and type, applied in directions such as silicon organic compounds, can solve the problems of complicated preparation process of aryl alkynes, difficult to obtain raw materials, and high production cost, and achieve the effects of easy industrial large-scale production, easy acquisition, and reduction of reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (S)-2-(6-methoxy-3-((triisopropylsilyl)ethynyl)naphthalen-2-yl)propanoicacid
[0020]
[0021] Add 0.69 g (S)-(+)-2-(6-methoxy-2-naphthyl)propionic acid, silver acetate 1 g, palladium acetate 69mg, and N-acetyl-L-phenylalanine into the reactor Acid 126 mg, sodium acetate 0.5 g, dichloroethane 5 ml, triisopropylsilylethynyl bromide 1 ml, and reacted at 60 ℃ for 16 h. After the reaction is completed, add acetic acid to acidify, extract three times with ethyl acetate, combine the organic phases, wash the organic phases with dilute hydrochloric acid and saturated brine, add an appropriate amount of anhydrous sodium sulfate to dry, filter to remove anhydrous sodium sulfate, petroleum ether: acetic acid Ethyl ester=10:1 was purified by column chromatography and distilled under reduced pressure to obtain 0.99g of product with a yield of 83.42%. 1 H NMR (501 MHz, CDCl 3 ) δ7.95 (s, 1H), 7.69 (s, 1H), 7.66 (d, J = 9.0 Hz, 1H), 7.13 (dd, J = 9.0, 2.5Hz, 1H), 7.06 (d, J = 2.5 Hz, ...
Embodiment 2
[0025] Example 2 Preparation of (S)-2-(4-isobutyl-2-((triisopropylsilyl)ethynyl)phenyl)propanoic acid
[0026]
[0027] Add 0.62 g of Levoprofen, 1 g of silver acetate, 69 mg of palladium acetate, 126 mg of N-acetyl-L-phenylalanine, 0.5 g of sodium acetate, 5 ml of dichloroethane, and triisopropyl in the reactor. 1 ml of silylethynyl bromide, react at 60 ℃ for 16 h. The other operation steps are the same as those in Example 1-1 to obtain 0.89 g of product (P1) with a yield of 76.72%. 1 H NMR (501 MHz, CDCl 3 ) δ 7.27(s, 1H), 7.23 (d, J = 8.0 Hz, 1H), 7.08 (d, J = 8.0 Hz, 1H), 4.39 (q, J = 7.0Hz, 1H), 2.42 (d, J = 7.0 Hz, 2H), 1.86 (dt, J = 13.5, 7.0 Hz, 1H), 1.50 (d, J = 7.0 Hz, 3H), 1.13 (s, 21H), 0.90 (d, J = 7.0 Hz, 6H); 13 C NMR (126 MHz, CDCl 3 ) δ 179.8, 140.4, 139.4, 133.3, 129.7, 126.2, 122.7, 104.9, 94.9, 44.7, 42.6, 29.9, 22.3, 18.6, 18.0, 11.3.
Embodiment 3
[0029] Preparation of (S)-8-((triisopropylsilyl)ethynyl)-1,2,3,4-tetrahydronaphthalene-1-carboxylic acid
[0030]
[0031] Add 0.53 g (S)-(-)-1,2,3,4-tetrahydro-1-naphthoic acid, silver acetate 1 g, palladium acetate 69 mg, and N-tert-butoxycarbonyl-L- Phenylalanine 140 mg, sodium acetate 0.5 g, dichloroethane 5 ml, triisopropylsilyl ethynyl bromide 1 ml, and reacted at 60 ℃ for 16 h. The other operation steps are the same as those in Example 1-1 to obtain 0.88 g of product with a yield of 82.24%. 1 H NMR(501 MHz, CDCl 3 ) δ 7.33 (d, J = 7.5 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 7.07(d, J = 8.0 Hz, 1H), 4.20 (d, J = 6.5 Hz, 1H), 2.83 (d, J = 17.0 Hz, 1H), 2.74 (m, 1H), 2.35 (d, J = 13.5 Hz, 1H), 2.05 – 1.94 (m, 1H), 1.80 (d, J =26.0 Hz, 2H), 1.12 (d, J = 2.0 Hz, 21H); 13 C NMR (126 MHz, CDCl 3 ) δ 180.4, 137.4, 135.0, 130.3, 129.4, 126.5, 124.3, 104.8, 95.9, 43.4, 29.1, 27.1, 19.5, 18.5, 11.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com