Multifunctional fusion protein for Her2+tumor and application thereof
A fusion protein, multifunctional technology, applied in antitumor drugs, fusion polypeptides, immunoglobulins, etc., can solve the problems of CD47 cell clearance, low efficacy, lack of red blood cells, etc., to enhance the function of inhibiting tumor growth and increase immune response , the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
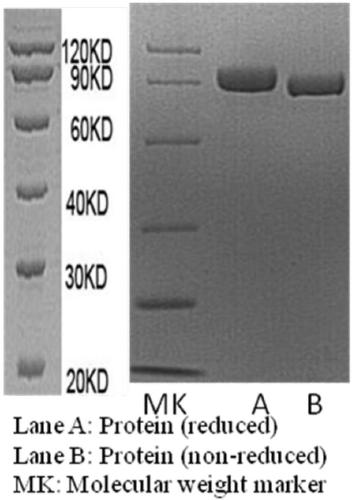
[0043] This example is the gene construction and production purification of the recombinant fusion protein.
[0044] According to the functional region amino acids of human SIRPα, anti-Her2scFv and IgG1Fc (positions 31-150 in the sequence shown in SEQ ID NO: 1, positions 1-119 in the sequence shown in SEQ ID NO: 2, positions in SEQ ID NO: 3 Bases corresponding to positions 1-107 in the shown sequence and positions 3-219 in the sequence shown in SEQ ID NO: 4 are synthesized with non-functional amino acid flexible fragments (sequence shown in SEQ ID NO: 5) or its repeat sequence) base-linked into a multifunctional fusion protein (SEQ ID NO: 6) gene, through restriction enzyme digestion and further cloning, and then transferred to the eukaryotic animal expression vector pcDNA3.1 (-). Finally, the fusion protein gene will be The vector was transfected into Chinese hamster ovary cells (CHO). The transfected cells were placed at 37°C, 5% CO 2 After culturing in an incubator, the su...
Embodiment 2
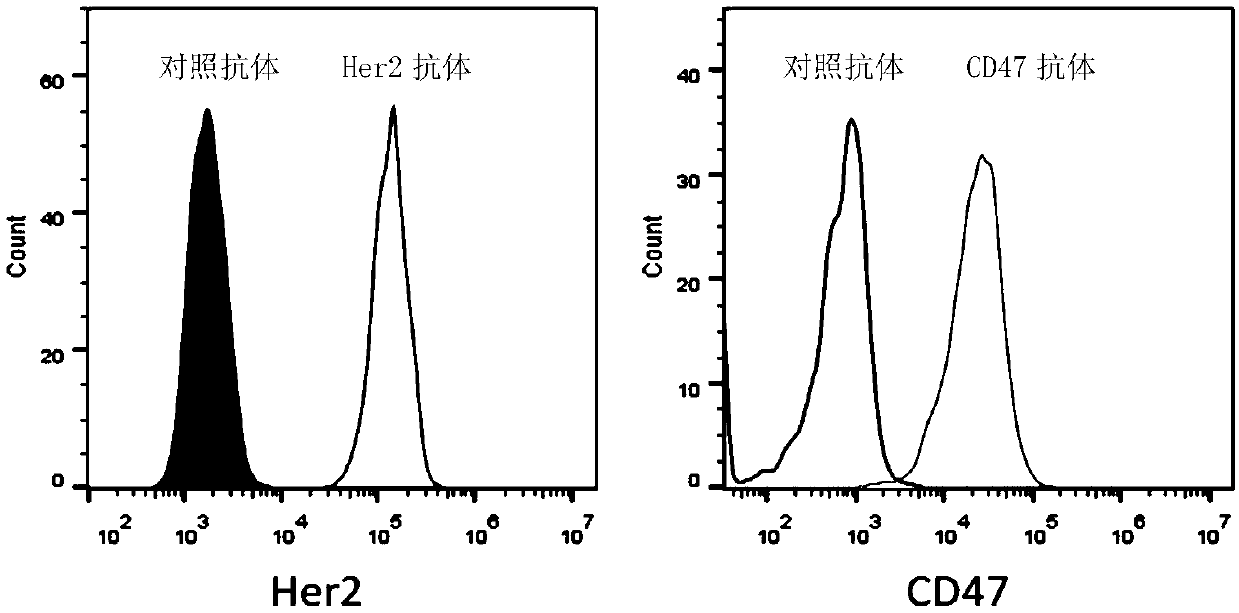
[0047] This example is an affinity test of the multifunctional recombinant fusion protein to Her2 positive tumor cells.
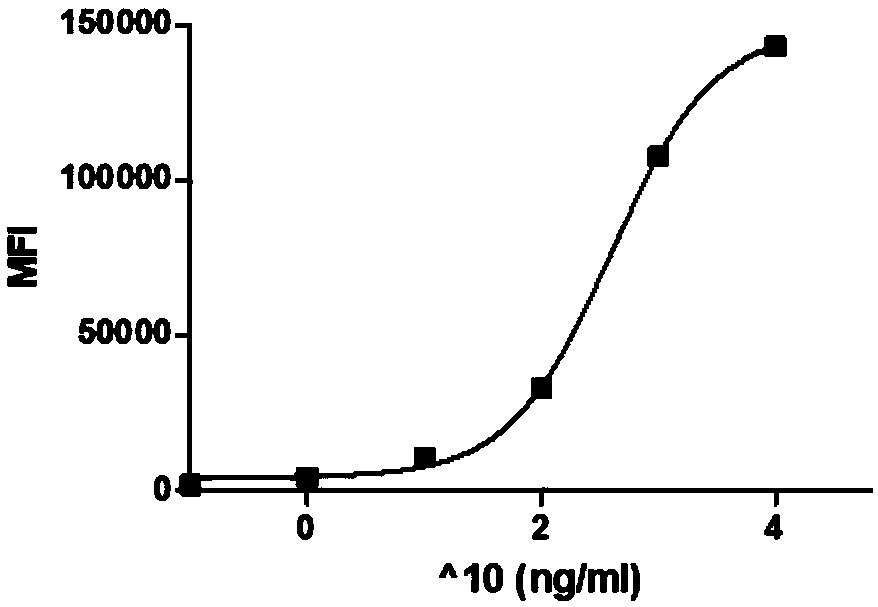
[0048] Determination of protein affinity curve for Her2+ cells: take Her2-positive SK-BR-3 tumor cells, 1×10 per well 4 Cells were placed in a 96-well plate in 0.1 ml of PBS, PE-labeled fusion proteins of different concentrations were added, mixed well, and left at room temperature for 0.5 hours. After the cells were collected, they were washed once with PBS, and finally PE fluorescence was measured and data analysis was performed by flow cytometry. image 3 Shown in , for Her2 positive cells, the fluorescence signal (MFI) of PE is positively correlated with the fusion protein concentration. This example proves that the fusion protein can bind to Her2-positive tumor cells, and proves that the fusion protein has good targeting to Her2.
Embodiment 3
[0050] This example shows the shielding effect of the multifunctional recombinant fusion protein on the CD47 signaling pathway.
[0051] Dilute 1 x 10 in 100 μl PBS 4 Her2-positive SK-BR-3 cells were transferred to a 96-well plate, and then different concentrations of fusion proteins were added, and left at room temperature for 20 minutes. After washing once, APC-labeled anti-human CD47 flow antibody was added for staining, and left at room temperature for 15 minutes. After washing, the fluorescence signal of APC and data analysis were measured by flow cytometer. Figure 4 Shown in is the signal intensity (APC fluorescence intensity) of CD47 antibody binding to Her2 positive cells analyzed by flow cytometry. It can be seen from the figure that the 200 ng / ml fusion protein almost completely shields the binding of CD47 and the antibody, that is, the fusion protein of the present invention can completely shield the CD47 signaling pathway, achieving the design purpose of this emb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com