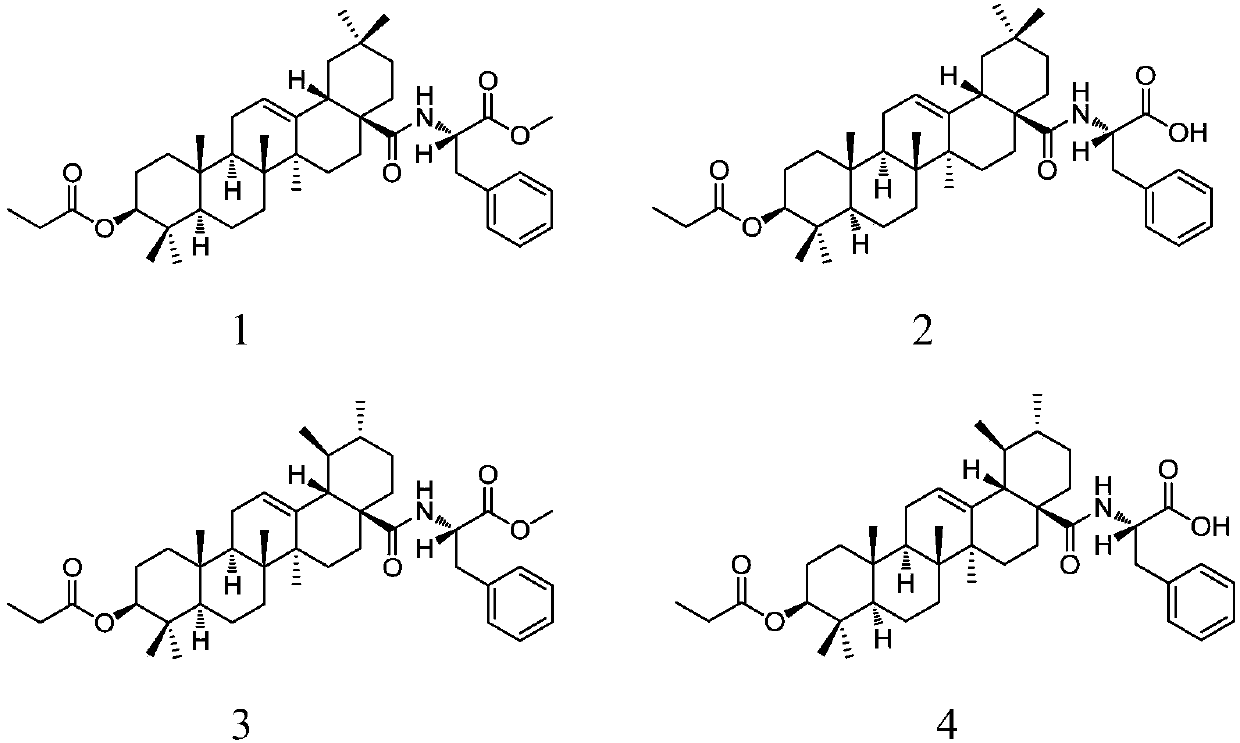
28-(L-phenylalanine)-pentacyclic triterpene derivatives as well as synthesis methods and application thereof
A synthesis method and technology of phenylalanine are applied in the directions of drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc. to achieve the effects of good proliferation inhibitory activity and good medicinal value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of compound 1
[0033]
[0034] 3-Propionyloxy-oleanolic acid (0.50 g, 1.0 mmol) was dissolved in CH 2 Cl 2 (10mL), add dropwise SOCl 2 (0.45mL), reflux at 72°C for 2h, the reaction solution was concentrated under reduced pressure, and washed with CH 2 Cl 2 (2×20mL) spin off SOCl 2 ; The resulting residue was dissolved in CH 2 Cl 2(20mL), add L-phenylalanine methyl ester (0.26g, 1.5mmol) and saturated aqueous sodium bicarbonate solution (1.25mL) successively under ice-bath conditions, stir and react at room temperature for 12h; the obtained reaction material is dispersed in 1mol / L HCl (100mL), CH 2 Cl 2 (3×30 mL), combined the organic layers, washed with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried to obtain a crude product. The crude product was separated by silica gel column chromatography (eluent: V 石油醚 :V 乙酸乙酯 =30:1to5:1), to obtain compound 1 (0.48g, 73.0%, white ...
Embodiment 2
[0036] Embodiment 2: the synthesis of compound 1
[0037] Dissolve 3-propionyloxy-oleanolic acid (0.50 g, 1.0 mmol) in a mixed solvent composed of MeOH and THF (5 mL each of MeOH and THF), add dropwise oxalyl chloride (0.45 mL), and reflux at 60 ° C for 1 h , the reaction solution was concentrated under reduced pressure, and MeOH (2×20mL) was used to spin off oxalyl chloride; the obtained residue was dissolved in MeOH (20mL), and L-phenylalanine methyl ester (0.26g, 1.5mmol), Saturated potassium carbonate aqueous solution (1.25mL), stirred and reacted at 50°C for 8h; the obtained reaction material was dispersed in 2mol / L HCl (100mL), extracted with EtOAc (3×30mL), combined organic layers, and washed with water and saturated saline respectively Washed, dried over anhydrous magnesium sulfate, filtered, and the filtrate was spin-dried to obtain a crude product. The crude product was separated by silica gel column chromatography (eluent: V 石油醚 :V 乙酸乙酯 =30:1 to 5:1), a white sol...
Embodiment 3
[0039] Embodiment 3: the synthesis of compound 2
[0040]
[0041] Compound 1 (0.45 g, 0.66 mmol) was dissolved in MeOH, THF and H 2 Mixed solvent composed of O (9mL, MeOH, THF and H 2 The volume ratio of O is 1:1:1), add 2mol / L sodium hydroxide solution (0.5mL / 30min, 1.5mL, 3.0mmol) under ice-bath conditions, stir and react at room temperature for 12h; Adjust the pH to 2 with HCl, spin off MeOH and THF under reduced pressure; the resulting residue was dispersed in EtOAc (100 mL), washed with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried to obtain a crude product. The crude product was separated by silica gel column chromatography (eluent: V 石油醚 :V 乙酸乙酯 =3:1to 1:1), compound 2 (0.41 g, 94%, white solid) was obtained.
[0042] Yield: 0.41g, 94%, white solid; R f =0.29(Petroleum ether:EtOAc=1:1).m.p.108-110℃. 1 H NMR (500MHz, CDCl 3 ):δ0.62,0.76(2s,each 3H,2×CH 3 ),0.84(s,6H,2×CH 3 ),0.87,0.88,1.10(3s,each 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com