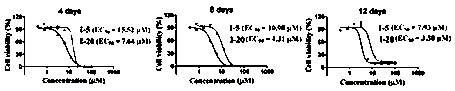5-benzylidene-2-phenyl thiazolone compound as well as preparation and application thereof
A technology of phenylthiazolone and benzylidene, which is applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve problems such as genome instability and cell cycle arrest, and achieves simple and easy preparation method, easily available raw materials, and strong tumor cells. Effect of proliferation inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 general formula (I) compound
[0039] Dissolve p-toluonitrile (59 mg, 0.5 mmol), thioglycolic acid (1 equiv), triethylamine (1 equiv) and the corresponding benzaldehyde derivative (1 equiv) in 10 mL of ethanol. The mixture was heated to reflux for 12 hours, and then the solvent was removed under reduced pressure, and the residue was separated by column chromatography to obtain the target compound.
[0040]
[0041] The following products are obtained:
[0042] ( Z )-5-benzylidene-2-( p -Methylphenyl)thiazole-4(5 H )-ketone (I-1), yellow solid (46 mg, 33%), melting point 190-192 °C. 1 H NMR (600 MHz, CDCl 3 ) δ 8.12 (d, J= 7.8 Hz, 2H), 8.05 (s, 1H),7.68 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.4 Hz, 2H), 7.37 (d, J = 7.8 Hz, 2H),2.48 (s, 3H). 13 C NMR (150 MHz, CDCl 3 ) δ 187.1, 183.4, 146.7, 138.1, 133.9, 131.0, 130.6, 130.0, 129.2, 129.2, 129.0, 126.6, 22.0. ESI-MS m / z : 280.0 [M+H] + .
[0043] ( Z )-5-(2-Methoxybenzyli...
Embodiment 2
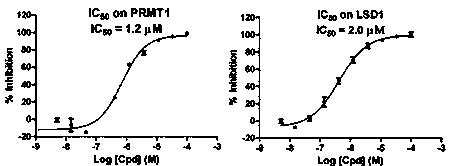
[0068] Example 2 The enzyme activity inhibitory activity of compounds was tested by radioisotope method.
[0069] 1. Prepare 1x assay buffer (improved Tris-HCl buffer); 2. Dilute the compound to the desired concentration in a 96-well plate; 3. Prepare protein solution, also use 1x assay buffer; 4. Dilute the substrate Add to 1x assay buffer to prepare substrate solution; 5. [ 3 H]-SAM was added to 1x assay buffer to prepare [ 3 H]-SAM solution; 6. Add SAM to 1x assay buffer to prepare a cold SAM solution; 7. Pipette 10 μL protein solution to a 96-well plate containing the compound; 8. Incubate at room temperature for 15 minutes; 9. Add 10 μL substrate solution to each well; 10. Add 10 μL [ 3 H]-SAM solution initiates the reaction; 11. Incubate at room temperature for 240 minutes. 12. Add 10 μL of cold SAM solution to each well to terminate the reaction; 13. Transfer 40 μL of the reaction mixture solution to the GF / B plate, and wash with triple-distilled water for 3 times in...
Embodiment 3
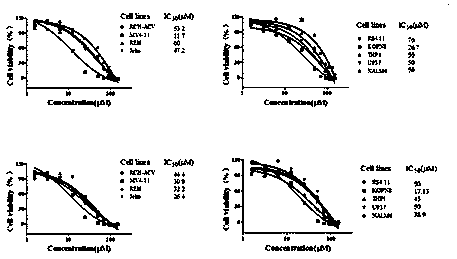
[0084] Example 3 Effects of Compounds on Cell Proliferation
[0085] 1. Test method
[0086] (1) Cell culture
[0087] The culture medium used for MV4-11, Jeko, KOPN8, RCH-ACV, REH, RS4.11, THP1, U937, NALM6 cell culture is RPMI 1640 + 10% fetal bovine serum, and in order to prevent bacterial contamination, 100 U / mL penicillin and 100 μg / mL streptomycin. The cells were cultured at 37°C and 5% CO2 saturated humidity, and the cells used in the experiment were all in the logarithmic growth phase.
[0088] (2) Detection of cell proliferation activity
[0089] Adjust cell concentration to 1 x 10 5 / mL and inoculated in a 24-well culture plate with a volume of 1 mL per well. Set up a control group and an experimental group. The control group was added with DMSO, and the experimental group was added with a small molecule inhibitor of PRMT5 activity to a final concentration of 0-100 μM. Three detection time points were set for MV4-11 cells, which were 4, 8 and 12 days respectiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com