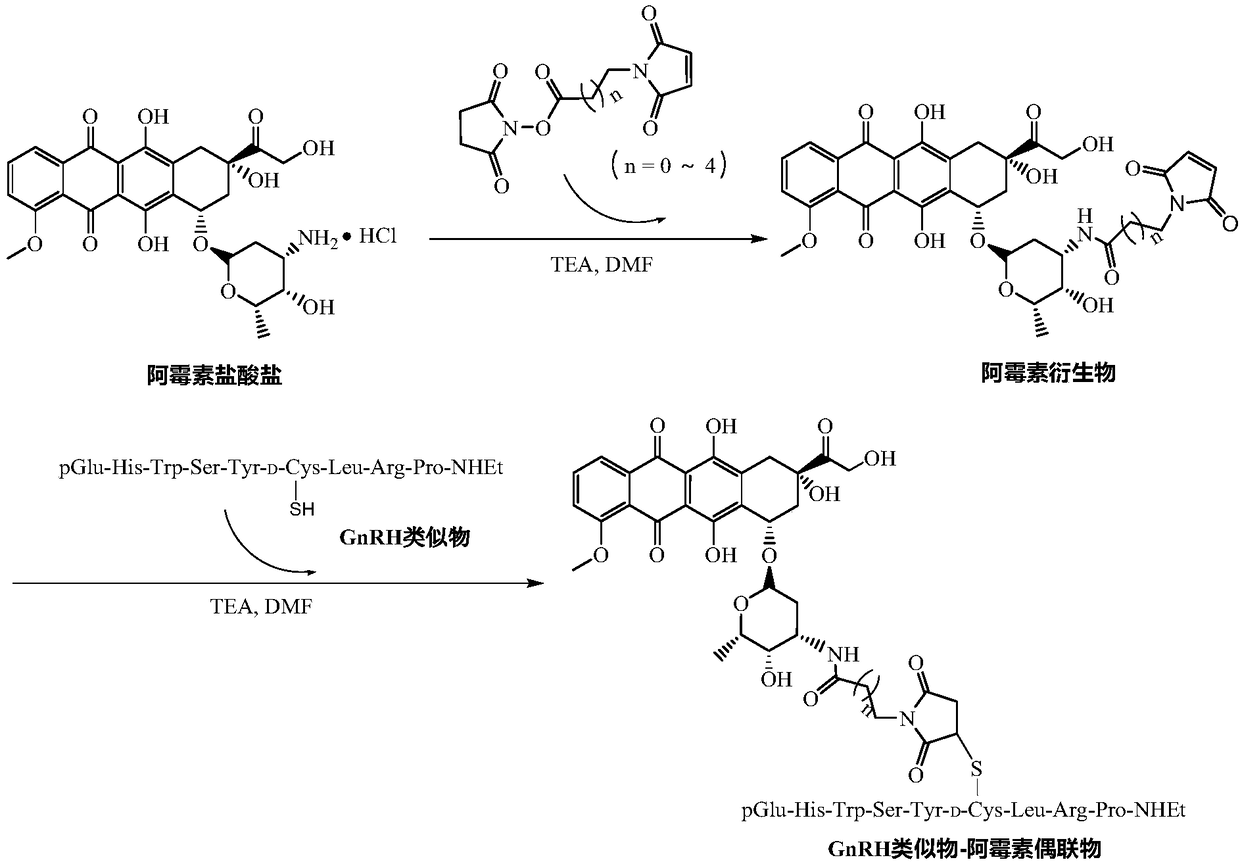
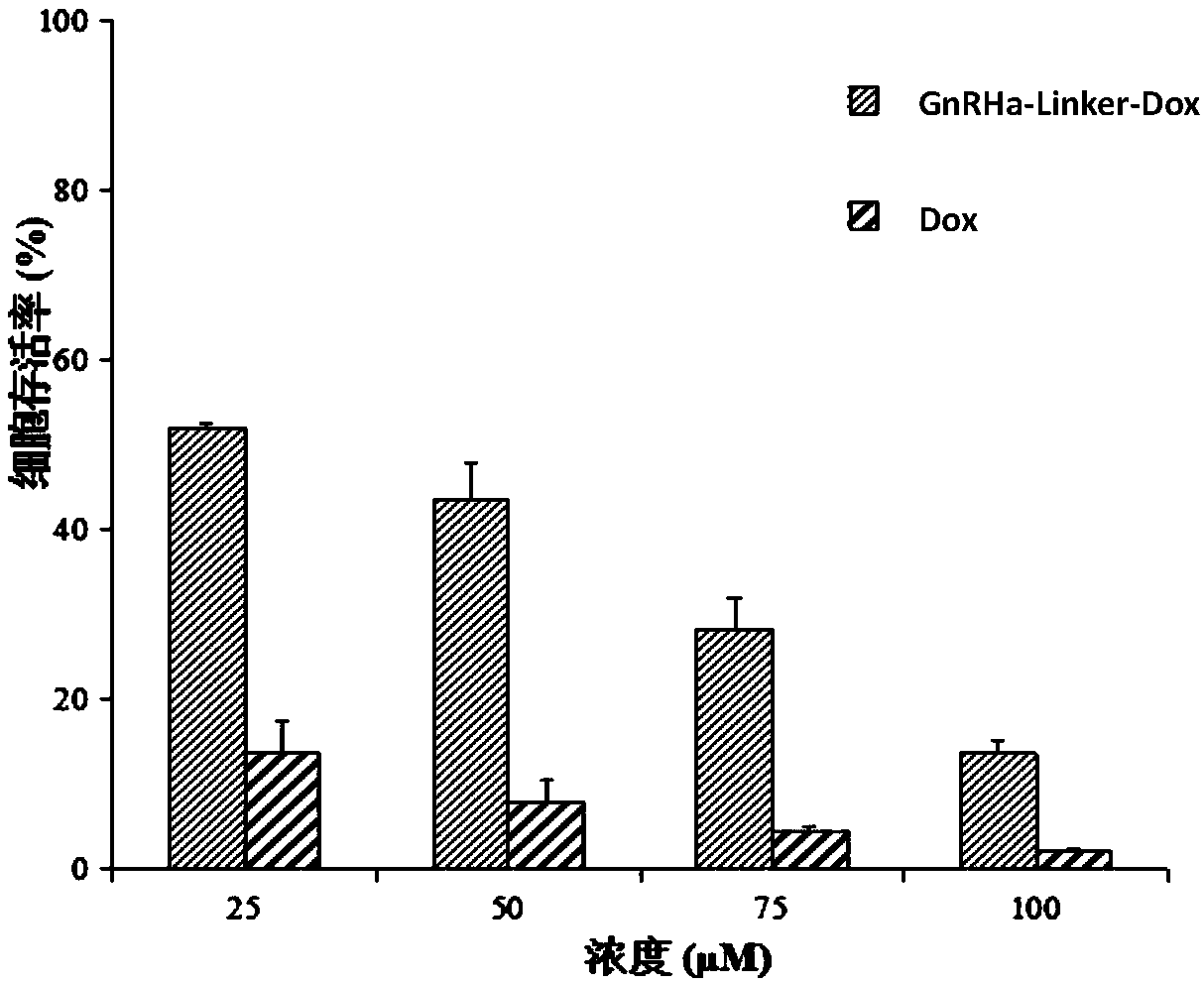
GnRH analogs-antineoplastic drug conjugate and preparation method and application thereof
A technology for anti-tumor drugs and conjugates, applied in the field of medicinal chemistry, can solve the problems of low stability of conjugates, toxic and side effects, and achieve good application prospects, reduce toxic and side effects, and enhance the effects of stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 [ D -Cys 6 -des Gly 10 -Pro 9 -NH 2 Synthesis of ]-GnRH:
[0066] 2-CTC resin (degree of substitution 1.3mmol / g, 5mmol) reacts with Fmoc-Pro-OH (15mmol) under the action of DIPEA to obtain Fmoc-Pro-2-CTC resin, wherein 2-CTC resin and DIPEA and Fmoc-Pro- The molar ratio of OH was 1:3:3; the obtained Fmo c-Pro-2-CTC resin was removed by piperidine / DMF solution with a volume concentration of 20%, and the ninhydrin test was positive, indicating that Fmoc protected The group was successfully removed; using the F moc / tBu cross-protection strategy, using DIC (22.5mmol) / HOBt (22.5mmol) as the condensation reagent, the remaining Fmoc was sequentially condensed from the C-terminal to the N-terminal according to the standard Merrifield peptide solid-phase synthesis method -amino acid (22.5mmol), wherein the molar ratio of Fmoc-Pro-CTC resin, condensation reagent DIC / HOBt and Fmoc-amino acid is 1:4.5 / 4.5:4.5; reaction time 2h, obtain [ D -Cys 6 -des Gly 10 -Pro ...
Embodiment 2
[0068] Reaction of doxorubicin with 3-maleimidopropionate hydroxysuccinimidyl ester (SMP)
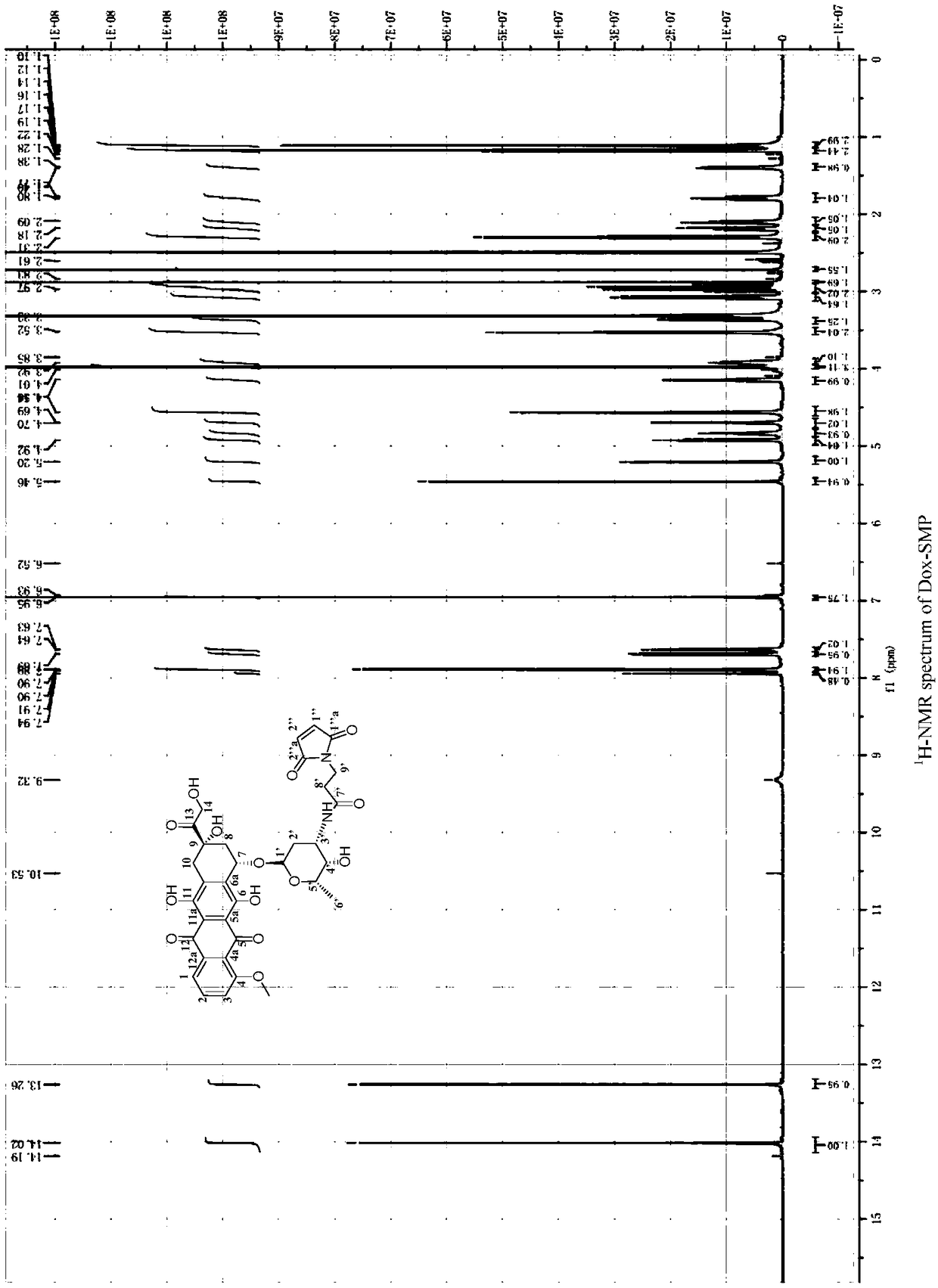
[0069] With reference to the records of Shi NQ, Gao W, Xiang B, et al. Enhancing cellular uptake of activable cell-penetrating peptide-doxorubicin conjugate by enzymatic cleavage [J]. Int J Nanomedicine.2012,7:1613–1621, weigh Dox·HCl (227mg, 0.39mmol) and SMP (114.5mg, 0.43mmol) were dissolved in anhydrous DMF (40mL), then TEA (125μL, 0.90mmol) was added, stirred at room temperature for 2h, and the reaction solution was settled with glacial ether (100mL) , after centrifugation, the precipitate was washed with glacial ether (50mL×3), and dried under vacuum at constant temperature to obtain 174.5mg of doxorubicin derivatives (Dox-SMP, yield 64%), which were analyzed by HPLC, ESI-MS and 1 H-N MR( figure 2 ) to be characterized.
[0070] HPLC analysis purity is 96%; ESI-MS: m / z, [M+Na] + : 717.6 (theoretical value), 717.2 (experimental value); 1 H-NMR (300Hz, DMSO-D6): The single peak...
Embodiment 3
[0071] Example 3 Synthesis of GnRHa-Linker-Dox conjugates:
[0072] GnRHa (33.6mg, 28μmol) obtained in Example 1 and Dox-SMP (20mg, 28μmol) obtained in Example 2 were dissolved in anhydrous DMF (10mL), and TEA (80μL, 577μmol) was added, wherein GnRHa and Dox-SMP The molar ratio with TEA is 1:1:21; N 2The reaction was stirred at room temperature under protection, and the reaction progress was monitored by HPLC. After the reaction was completed, the reaction solution was settled with glacial ether (50mL), and after centrifugation, the precipitate was washed with glacial ether (20mL×3), and finally purified by semi-preparative high performance liquid chromatography and freeze-dried. Finally, the target conjugate GnRHa-Linker-Dox was obtained. ESI-MS: m / z, [M+H] + : 1895.0 (theoretical value), 1894.9 (experimental value); [M+2H] 2+ : 948.0 (theoretical value), 947.8 (experimental value).
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com