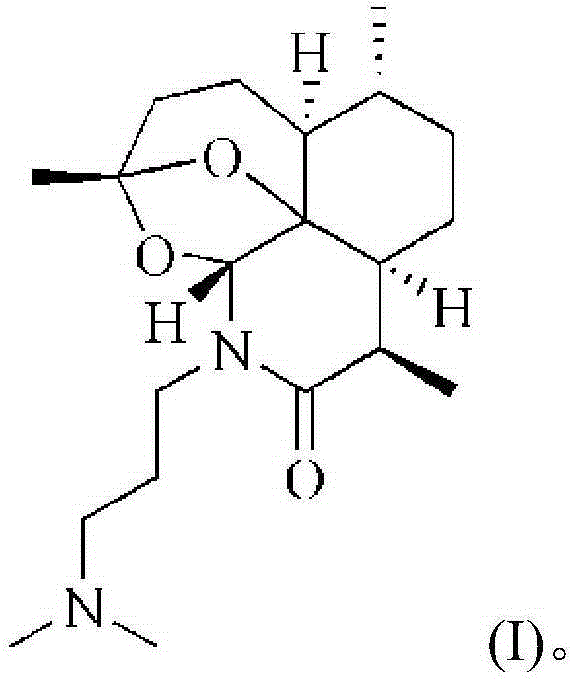
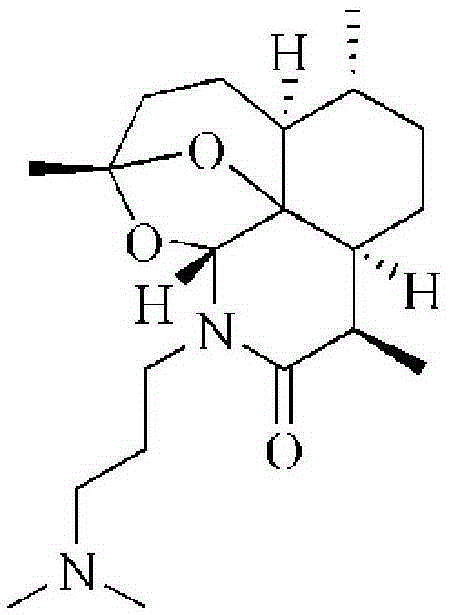
Artemisinin derivative, synthesis method and applications thereof
A synthesis method and reaction technology, applied in the field of medicine, can solve problems such as undiscovered and achieve good medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
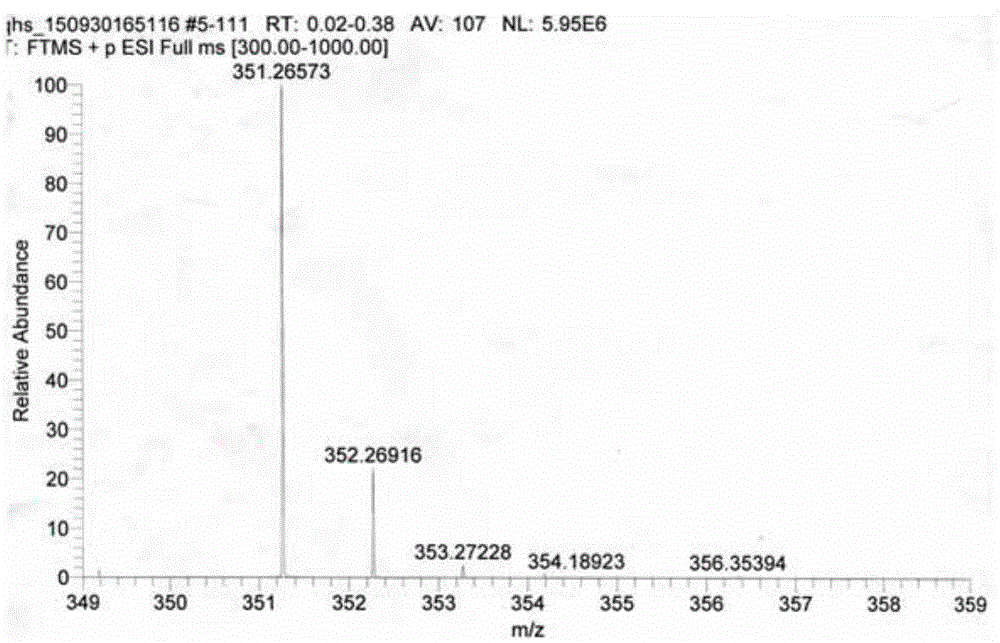
[0024] 1) Take 410mg (1.44mmol) of artemisinin and 30ml of methanol in a 100ml round bottom flask, stir for 5 minutes under ice bath, add 423mg (2.88mmol) of N,N-dimethyl-1,3-di Aminopropane, under the protection of nitrogen, react at 0°C for 24 hours, evaporate the solvent to dryness after the reaction, and collect the residue;
[0025] 2) Dissolve the residue collected in the previous step in 30ml of dichloromethane, then add 550mg (2.88mmol) p-toluenesulfonic acid, react at room temperature, follow the reaction with TLC, and evaporate the solvent after the reaction is completed (about 40 hours) , extracted three times with 5w / w% sodium bicarbonate solution, then extracted with water, collected the organic phase, dried with anhydrous sodium sulfate and evaporated to dryness to obtain an oily substance;
[0026] 3) The oil obtained in the previous step was purified by silica gel column chromatography (eluting solvent: petroleum ether: dichloromethane = 1:1, volume ratio) to o...
Embodiment 2
[0035] 1) Take 410mg (1.44mmol) of artemisinin, 15ml of ethanol and 15ml of dichloromethane in a 100ml round bottom flask, stir for 5 minutes under ice bath, add 211mg (1.44mmol) of N,N-dimethyl- 1,3-diaminopropane, under the protection of nitrogen, react at 0°C for 24 hours, evaporate the solvent to dryness after the reaction, and collect the residue;
[0036] 2) Dissolve the residue collected in the previous step in 20ml of dichloromethane, add 275mg (1.44mmol) p-toluenesulfonic acid, react at room temperature, track the reaction with TLC, and use 5w / w% Sodium carbonate solution was extracted three times, and then extracted with water, the organic phase was collected, dried with anhydrous sodium sulfate, and evaporated to dryness to obtain an oily substance;
[0037] 3) The oil obtained in the previous step was purified by silica gel column chromatography (eluting solvent: petroleum ether: dichloromethane = 1:2, volume ratio) to obtain the target product (60 mg, yield 11.9%)...
Embodiment 3
[0039]1) Take 410mg (1.44mmol) of artemisinin, 5ml of n-butanol, 5ml of hexanol and 15ml of chloroform in a 100ml round bottom flask, stir for 5 minutes under ice bath, add 634mg (4.32mmol) of N,N-di Methyl-1,3-diaminopropane, under the protection of nitrogen, react at 0°C for 18 hours, evaporate the solvent to dryness after the reaction, and collect the residue;
[0040] 2) Dissolve the residue collected in the previous step in 40ml of chloroform, add 825mg (4.32mmol) p-toluenesulfonic acid, react at room temperature, track the reaction with TLC, extract three times with ammonia water after the reaction is completed (about 30 hours), and then water Extraction, collecting the organic phase, drying with anhydrous sodium sulfate and evaporating the solvent to obtain an oil;
[0041] 3) The oil obtained in the previous step was purified by silica gel column chromatography (eluting solvent: petroleum ether:dichloromethane=1:0.5, volume ratio) to obtain the target product (120 mg, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com