Application of UiO-66 coated with rhodamine 6G in fluorescence detection of ferric ions
A fluorescence detection, uio-66 technology, applied in fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of small linear range and poor applicability between fluorescence intensity and iron ion concentration, and achieve good selectivity, easy storage and stability good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
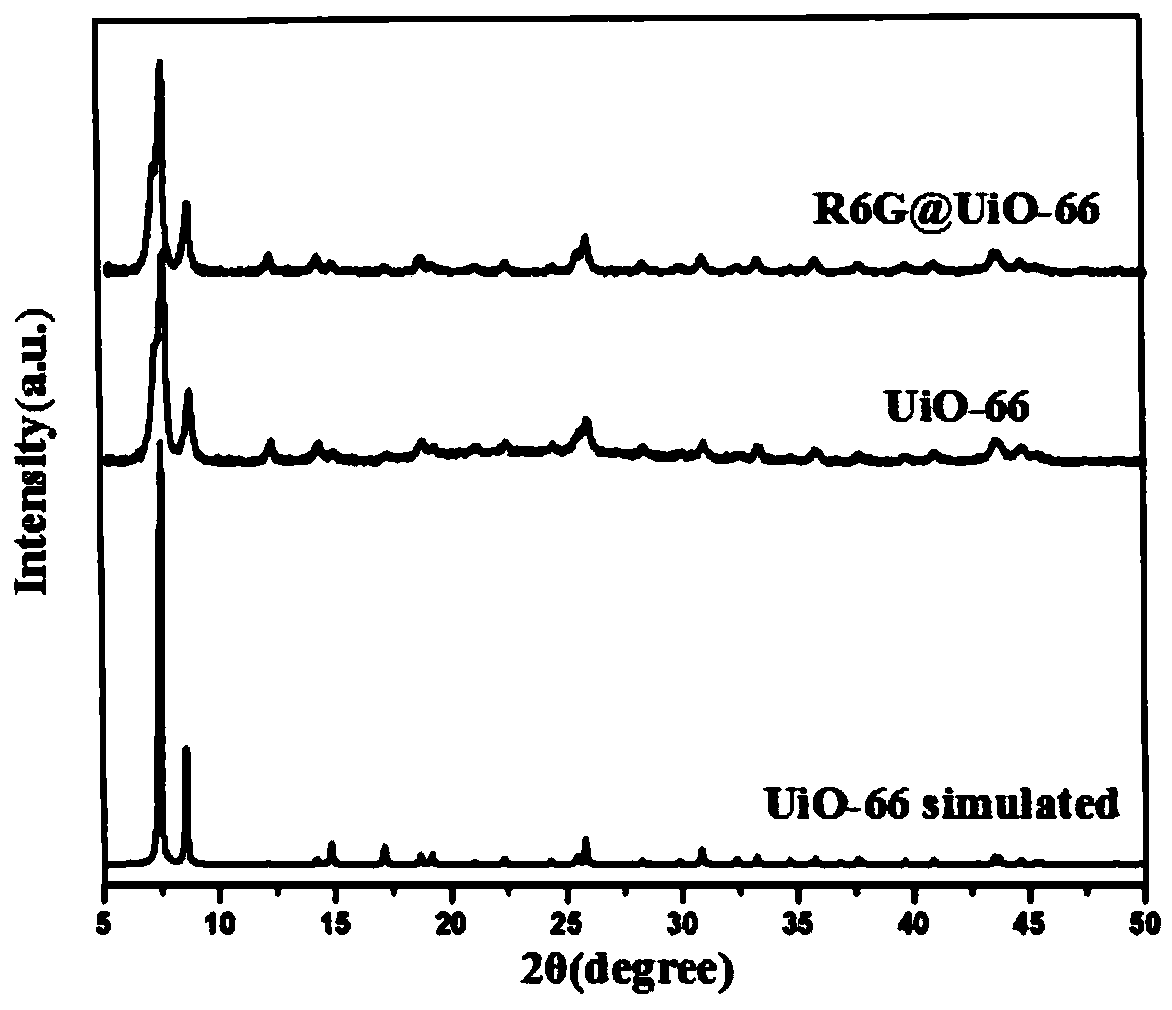
[0031] 1. Synthesis of UiO-66
[0032] ①Take a 50mL beaker, pipette 14mL of DMF into the beaker, then pipette 2.5mL of glacial acetic acid into the beaker, then add 5mM zirconium chloride into the beaker, and sonicate until the zirconium chloride dissolves.
[0033] ②Take a 10mL beaker, pipette 4mL of DMF into the beaker, then add 5mM terephthalic acid, and sonicate until dissolved.
[0034] ③Transfer the solution in step ② into the beaker of step ①, stir for 5 minutes, then transfer to a 50mL polytetrafluoroethylene autoclave, heat up the oven at 20°C per hour, keep it at 120°C for 36h, and then directly cool down , centrifuged at 12,000rpm for 3 minutes, washed the solid powder with DMF 3 times, soaked overnight, then centrifuged the powder, washed 3 times with methanol, soaked overnight, collected the powder by centrifugation, and dried overnight in vacuum at room temperature to obtain a white The powder is UiO-66.
[0035] 2. Synthesis of R6G@UiO-66
[0036] UiO-66 (15m...
Embodiment 2
[0038] Embodiment 2 ratiometric fluorescence method detects Fe 3+
[0039] (1) Add R6G@UiO-66 to distilled water to prepare a 50mg / L fluorescent probe solution.
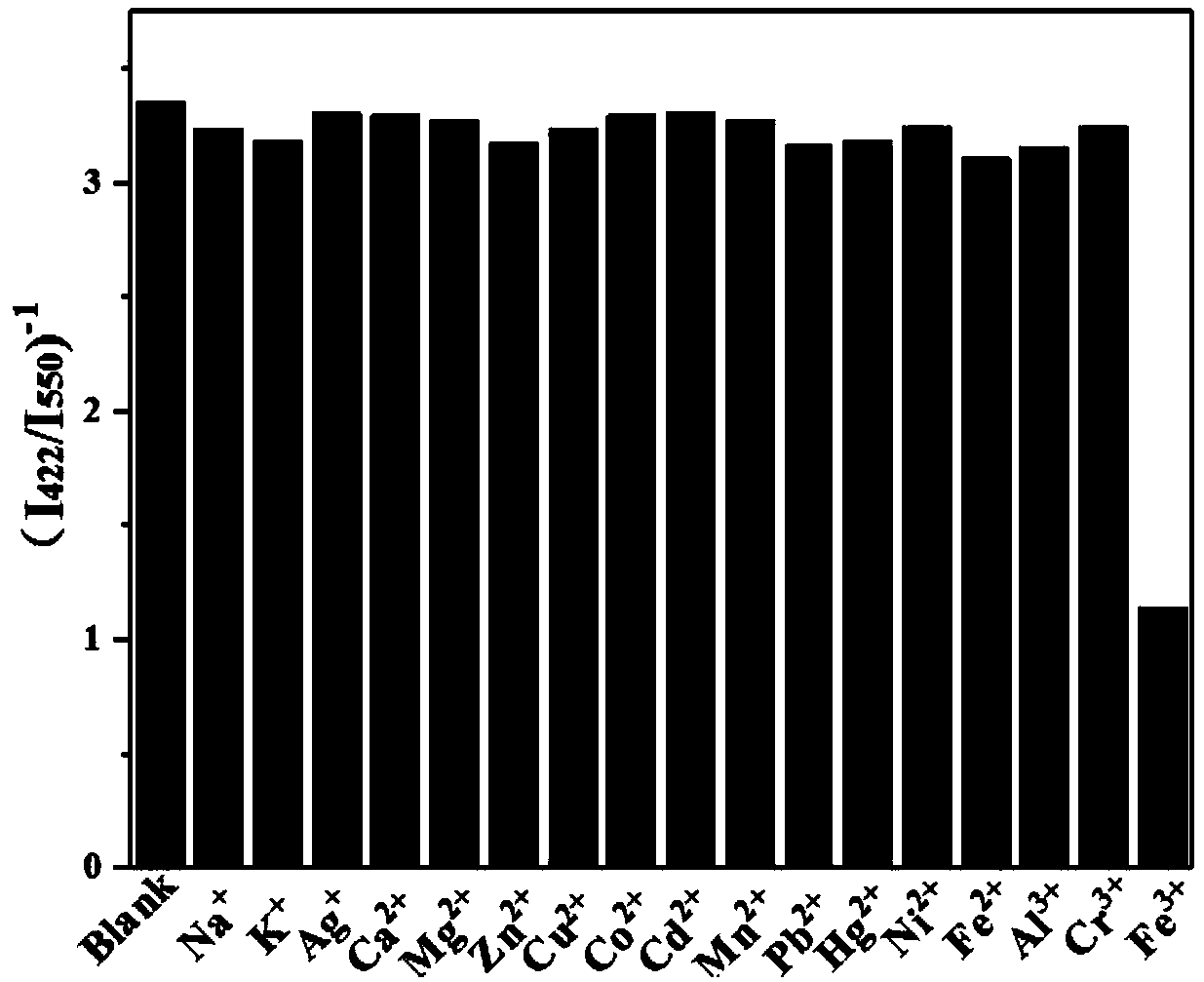
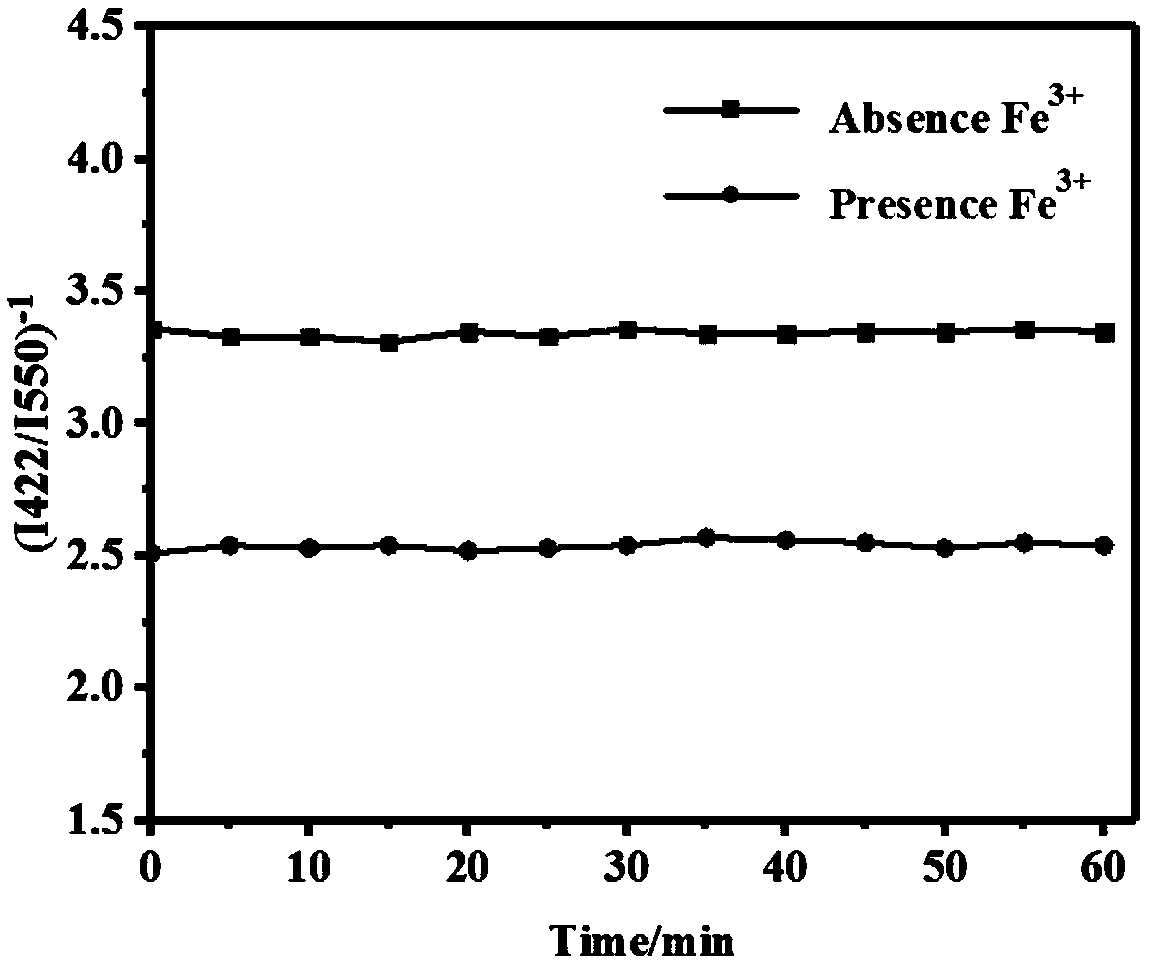
[0040] (2) Sample solution: configure a series of different concentration levels (10 -7 M, 10 -6 M, 10 -5 M, 10 -4 M, 10 -3 M, 10 -2 M) Fe 3+ The standard solution and other metal cation solutions with a concentration of 300 μM are ready for use.
[0041] (3) Get 2mL of the fluorescent probe solution of step (1) in a four-sided light-transmitting quartz cuvette, then add the sample solution of step (2), detect the fluorescence intensity with a fluorescence spectrometer, and set the slit width of the fluorescence spectrometer 10nm, set the excitation light wavelength to 365nm, and detect the emission peak fluorescence intensity at the wavelengths of 422nm (corresponding to the fluorescence of UiO-66) and 550nm (corresponding to the fluorescence of R6G).
[0042] The results showed that the addition of Fe 3+ ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com