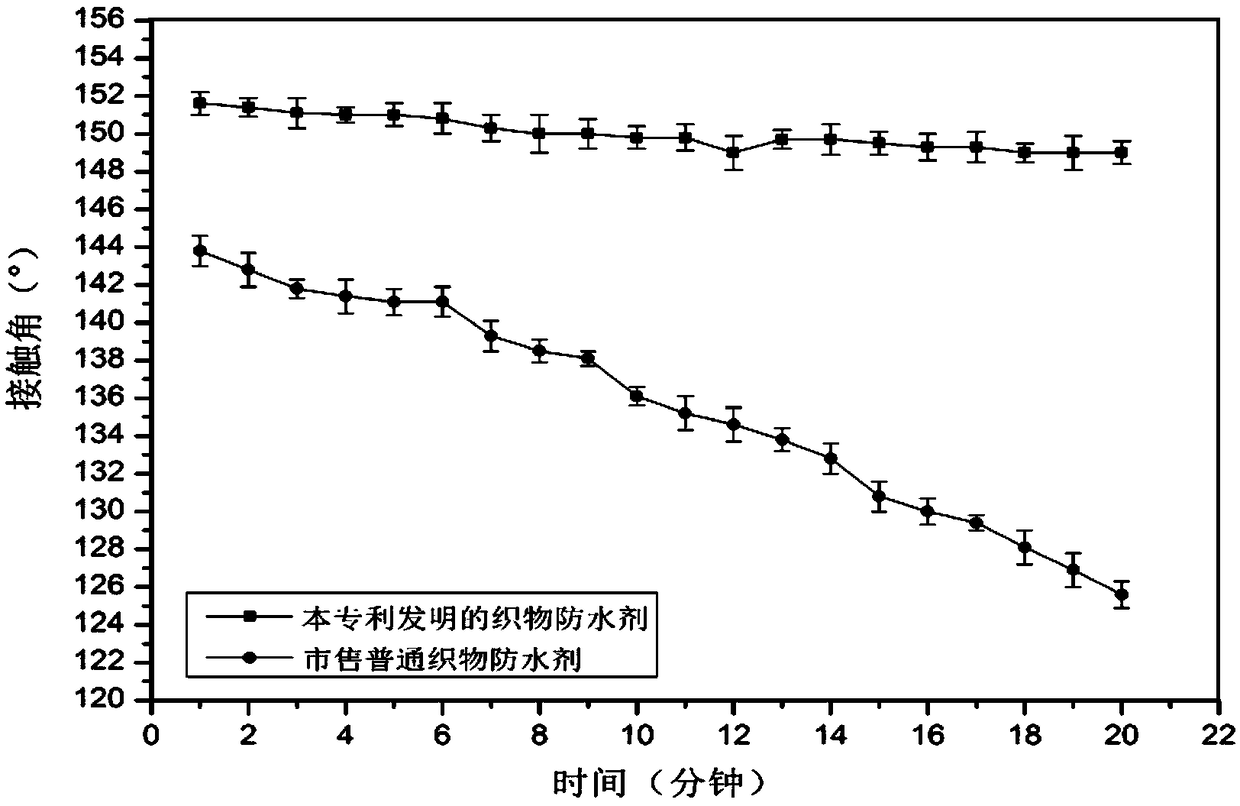
Durable fabric waterproofing agent and preparation method thereof
A water repellent, durable technology, applied in plant fibers, textiles and papermaking, fiber processing, etc., can solve the problems of poor waterproof effect, molecular conformation rearrangement, poor waterproof effect, etc. It is suitable for industrial production and popularization and application. The effect of low surface energy, durable water repellency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Preparation of trifluorooctyl p-acryloyloxybenzoate functional monomer
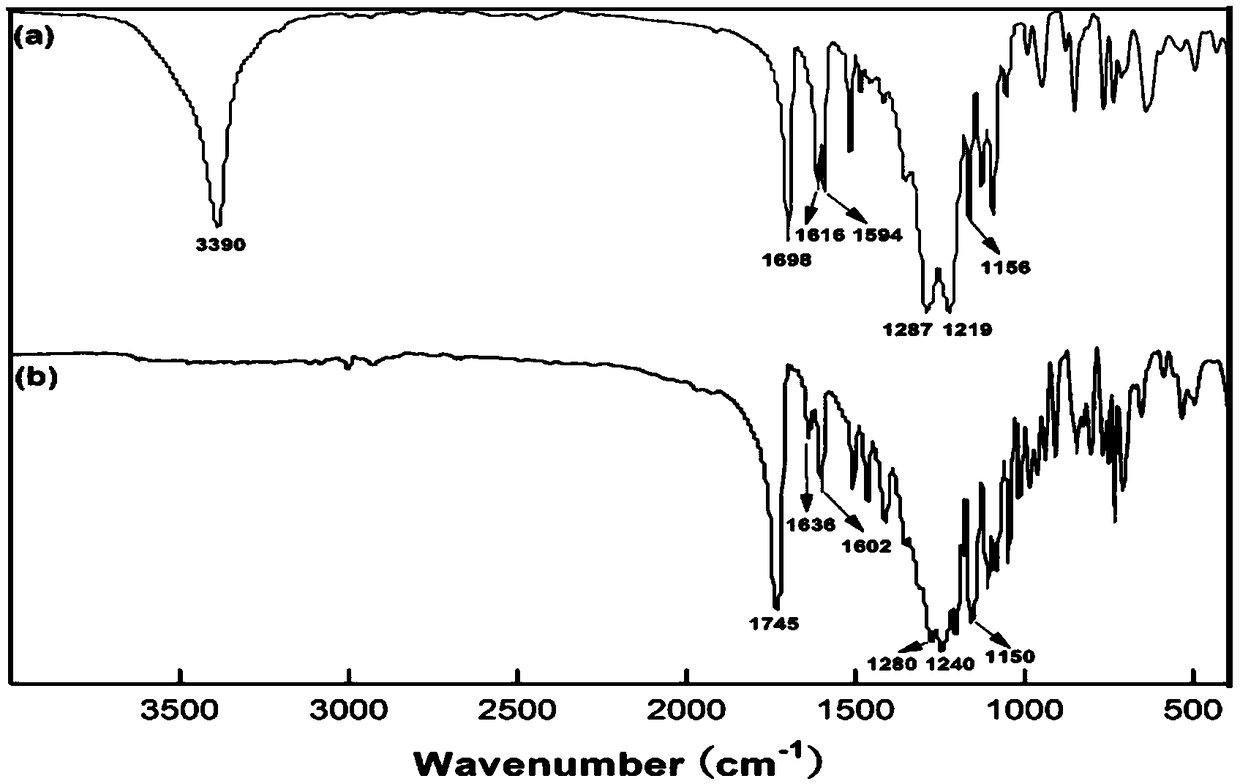
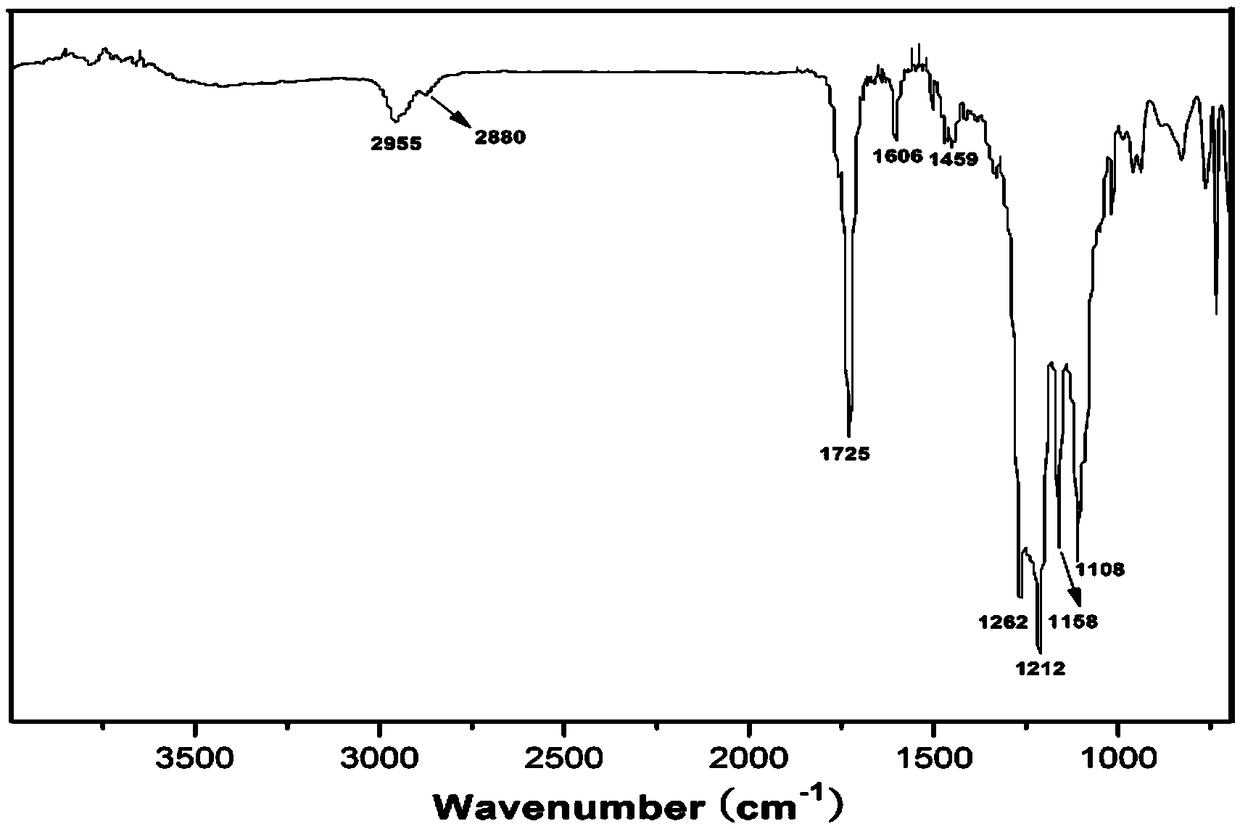
[0045] In a three-necked flask equipped with magnetic stirring, water separation device, reflux condenser and thermometer, add 4.15 grams of p-carboxybenzoic acid, 11.01 grams of tridecafluorooctyl alcohol, 0.8 grams of p-toluenesulfonic acid and 100 grams of xylene in sequence, and heat to 130° C., and reacted in a nitrogen atmosphere for 12 hours. After the reaction, the reaction solution was cooled to room temperature (20°C), filtered with suction, washed with hot water at 70°C, and dried (placed in a vacuum drying oven at a temperature of 50°C) to obtain a white solid The intermediate product of trifluorooctyl p-hydroxybenzoate.
[0046] In a three-necked flask equipped with a magnetic stirrer, a thermometer, a constant pressure dropping funnel, and a reflux condenser, add 20 grams of dichloromethane, 1.21 grams of triethylamine, and 4.84 grams of trifluorooctyl p-hydroxybenzoate in sequence,...
Embodiment 2
[0060] 1. Preparation of trifluorooctyl p-acryloyloxybenzoate functional monomer
[0061] In a three-necked flask equipped with magnetic stirring, water separation device, reflux condenser and thermometer, add 4.0 grams of p-carboxybenzoic acid, 11.0 grams of tridecafluorooctyl alcohol, 0.7 grams of p-toluenesulfonic acid and 98 grams of xylene in sequence, and heat to 130° C., and reacted in a nitrogen atmosphere for 12 hours. After the reaction, the reaction solution was cooled to room temperature (20°C), filtered with suction, washed with hot water at 70°C, and dried (placed in a vacuum drying oven at a temperature of 50°C) to obtain a white solid The intermediate product of trifluorooctyl p-hydroxybenzoate.
[0062] In a three-necked flask equipped with magnetic stirring, a thermometer, a constant pressure dropping funnel, and a reflux condenser, add 18 grams of dichloromethane, 1.21 grams of triethylamine, and 4.84 grams of tridefluorooctyl p-hydroxybenzoate in sequence,...
Embodiment 3
[0070] 1. Preparation of trifluorooctyl p-acryloyloxybenzoate functional monomer
[0071] In a three-necked flask equipped with magnetic stirring, water separation device, reflux condenser and thermometer, add 4.2 grams of p-carboxybenzoic acid, 11.3 grams of tridecafluorooctyl alcohol, 0.9 grams of p-toluenesulfonic acid and 102 grams of xylene successively, and heat to 150° C., and reacted in a nitrogen atmosphere for 24 hours. After the reaction, the reaction solution was cooled to room temperature (20°C), filtered with suction, washed with hot water at 70°C, and dried (placed in a vacuum drying oven at a temperature of 50°C) to obtain a white solid The intermediate product of trifluorooctyl p-hydroxybenzoate.
[0072] In a three-necked flask equipped with a magnetic stirrer, a thermometer, a constant pressure dropping funnel, and a reflux condenser, add 22 grams of dichloromethane, 1.24 grams of triethylamine, and 4.88 grams of tridefluorooctyl p-hydroxybenzoate in sequen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com