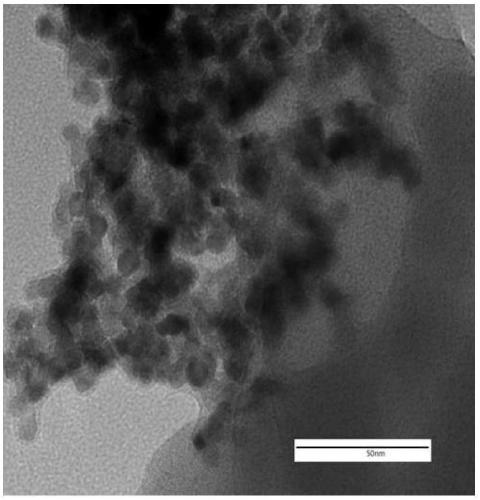
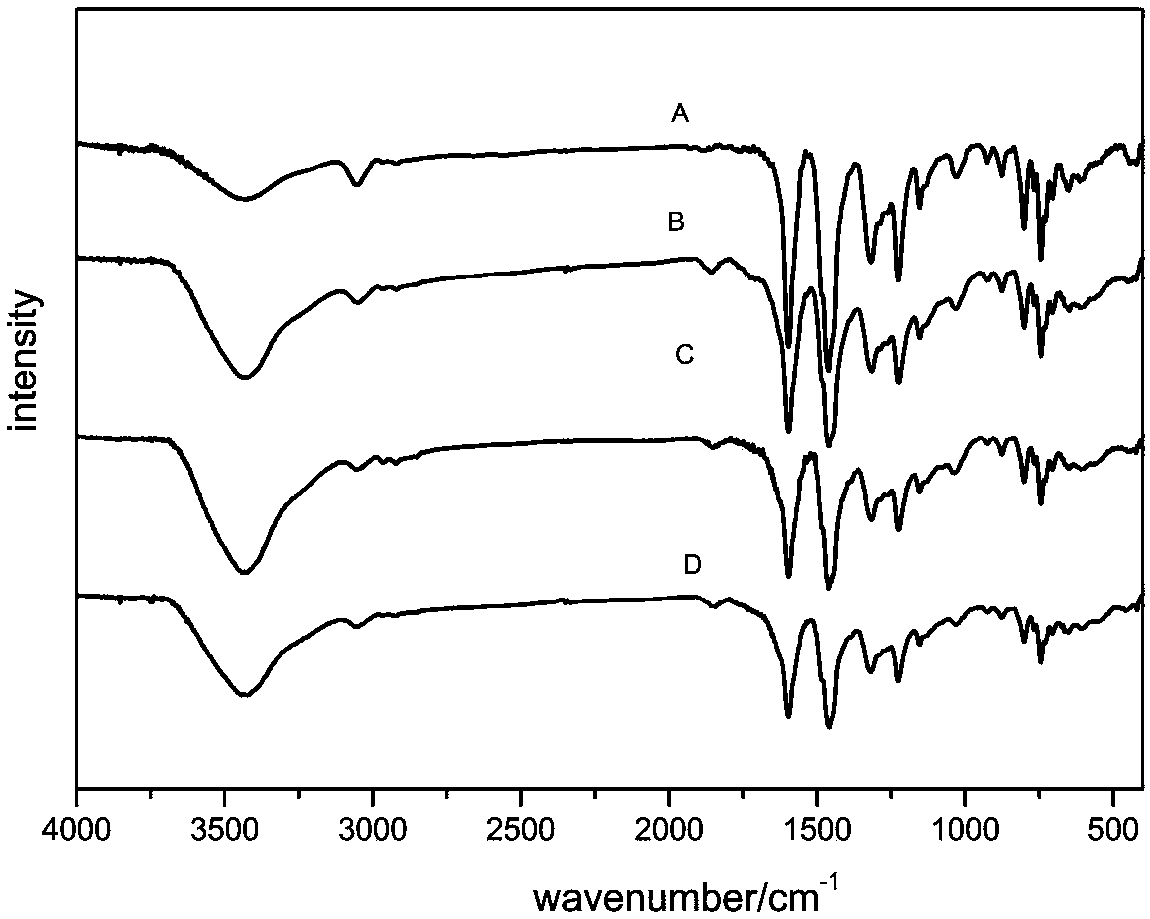
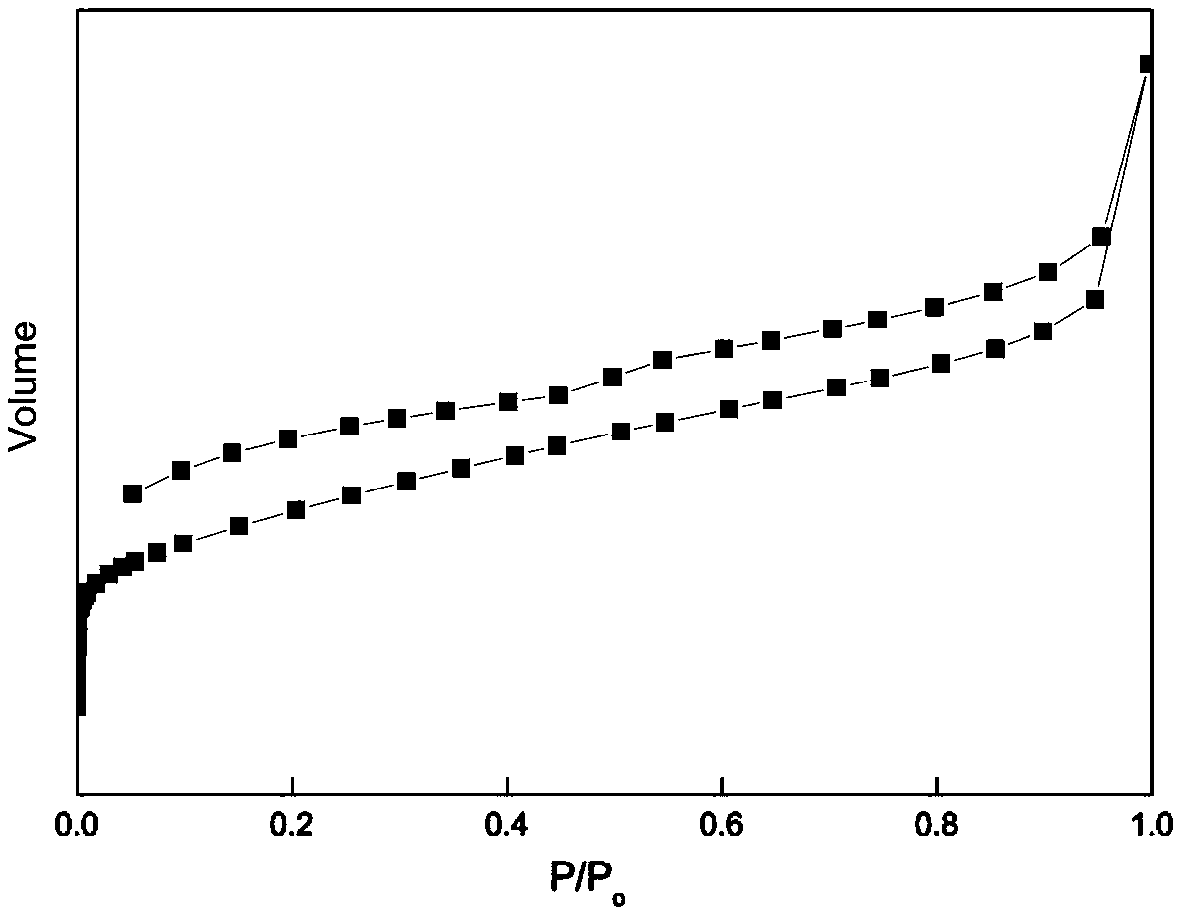
Carbazolyl porous organic polymer-carried transition metal oxide catalyst and application thereof
A transition metal and carbazole-based technology, applied in organic compound/hydride/coordination complex catalysts, preparation of organic compounds, physical/chemical process catalysts, etc., can solve the problem of insufficient activity of active centers and loss of active components and other problems, to achieve the effect of good repeated use performance, high activity and stability, and long service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 material A
[0024] 1) Weigh 2.0g of ferric chloride, replace it with nitrogen, add 70ml of anhydrous chloroform, then add dropwise 0.8g of 1,3,5-tris(9-carbazolyl)benzene dissolved in 70ml of anhydrous chloroform, Stirring at room temperature for 36 hours, washing, suction filtration, concentrated hydrochloric acid washing, Soxhlet extraction, and vacuum drying to obtain a carbazole-based porous organic polymer.
[0025] 2) Weigh 0.2g of carbazole-based porous organic polymer, add 40mg of cobalt acetate tetrahydrate, add 5ml of ethanol, and stir at 40°C for 24h;
[0026] 3) After impregnation, ethanol was removed by a rotary steamer, dried in an oven at 80°C for 12h, and calcined in a muffle furnace at 300°C for 3h to obtain material A.
Embodiment 2
[0027] The preparation of embodiment 2 material B-E
[0028] The preparation method of material B-E is the same as that of material A, the difference lies in the selection of metal salt, loading and calcination temperature. The obtained materials are listed in Table 1.
[0029] Table 1 Metal salts, loads and calcination temperatures used in materials B-E
[0030] Material
Embodiment 3
[0031] The preparation of embodiment 3 material F
[0032] 1) Weigh 2.0g of ferric chloride, replace it with nitrogen, add 70ml of anhydrous chloroform, then add dropwise 0.8g of 1,3,5-tris(9-carbazolyl)benzene dissolved in 70ml of anhydrous chloroform, Stirring at room temperature for 36 hours, washing, suction filtration, concentrated hydrochloric acid washing, Soxhlet extraction, and vacuum drying to obtain a carbazole-based porous organic polymer.
[0033] 2) Weigh 0.2g of carbazole-based porous organic polymer, add 80mg of manganese acetate tetrahydrate, add 5ml of ethanol, and stir at 40°C for 24h;
[0034] 3) After the impregnation, ethanol was removed by a rotary steamer, dried in an oven at 80°C for 12h, and calcined in a muffle furnace at 400°C for 3h to obtain material F.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com