Method for detecting cationic degree of synthetic polymer for drilling fluid
A technology for synthesizing polymers and cationic degrees, which is applied in the field of analytical chemistry, can solve the problems of low accuracy of cationic degree measurement results, achieve the effect of weakening ion-pair repulsion, improving accuracy, and avoiding low measurement results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
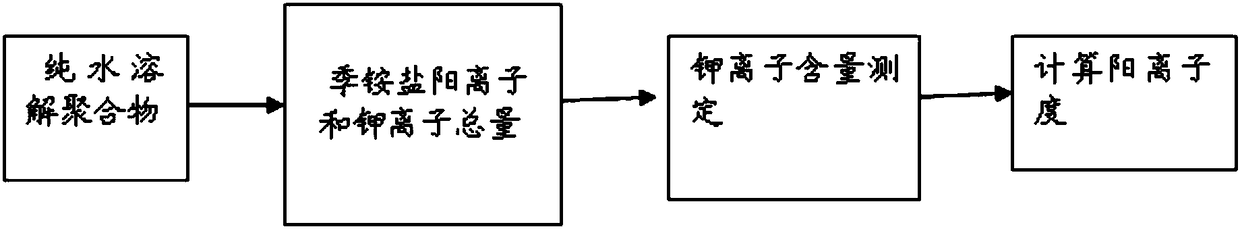
Image
Examples
Embodiment 1
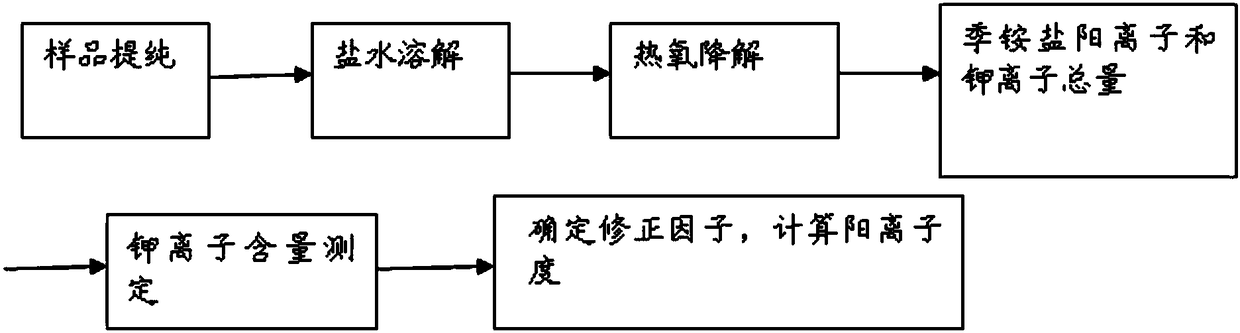
[0059] (1) Weigh 1.00g of acrylamidopropyltrimethylammonium chloride-acrylamide (AMPTA-AM) binary cationic polymer sample, soak in 100mL 90% ethanol solution for 2h, wash to remove small molecular ammonium ions and other For water-soluble impurities, filter with a layer of medium-speed qualitative filter paper, and wash the sample on the surface of the filter paper three times with 30 mL of 90% ethanol to remove residual impurities on the surface of the sample, dry at 105±3°C for 2 hours, cool to room temperature, and set aside .
[0060] (2) Weigh the purified sample m 1 =0.2000g (accurate to 0.0001g), add 8.0g of sodium chloride and distilled water to fully dissolve.
[0061] Then add 1mL hydrogen peroxide and 2-3 drops of dilute hydrochloric acid (volume ratio 1:1), heat at 80°C for 10min, cool to room temperature after fully degraded, and then adjust and control with acetic acid-sodium acetate buffer solution with a pH value of 3.6 pH value to 3.0 ~ 4.0, and then accurat...
Embodiment 2~4
[0070] The measurement sample objects in Example 1 were changed to binary copolymers of different cationic monomers and terpolymers and tetrapolymers containing anionic groups, and other test conditions were controlled to be unchanged. The measurement results are shown in Table 3 below.
[0071] According to the above test method, the theoretical cationic degree and the measured cationic degree of a series of polymers with different anionic group contents synthesized in the laboratory are compared, and the query chart of the correction factor of the cationic degree is summarized through data analysis.
[0072] Table 3 The results of the cationic degree measurement of different cationic polymers
[0073]
[0074] Note: DMD-AM is dimethyl diallyltrimethylammonium chloride-acrylamide binary copolymer; AMPTA-AM-AMPS is acrylamidopropyltrimethylammonium chloride-acrylamide-2- Acrylamide-2-methylpropanesulfonic acid terpolymer; AMPTA-AM-AMPS-AA is acrylamidopropyltrimethylammoniu...
Embodiment 5
[0110] Example 5 Molecular structure influence test on the determination of cationic degree
[0111] Zwitterionic polymers also contain a certain amount of anion -SO 3 2- Group and -COO - group, with the increase of the molar content of anionic groups in the polymer, the determination method of sodium tetraphenylborate has a large deviation, when the polymer-SO 3 2- The group molar content is 20%, -COO - When the group molar content is 20%, the measurement deviation of the sodium tetraphenylborate method is as high as 93%. It can be seen that the sodium tetraphenylborate method has shown great incompatibility. For this reason, the application uses a certain amount of chemical treatment agent (salts) to carry out charge shielding to anionic groups, remove or weaken the repulsion of anionic groups to tetraphenylborate ions, that is, add an appropriate amount of inorganic salt (NaCl) in water Dissolve the polymer, and use the same ion effect and the flocculation of inorganic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com