Method and detection kit for identifying degenerative osteoarthropathy biomarkers
A technology for biomarkers and osteoarthritis, applied in the field of medicine, can solve the problems of unsuitable for routine purposes, cumbersome processing process, large volume of serum samples, etc., and achieve the effects of simplified operation, short analysis time, and simple pretreatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
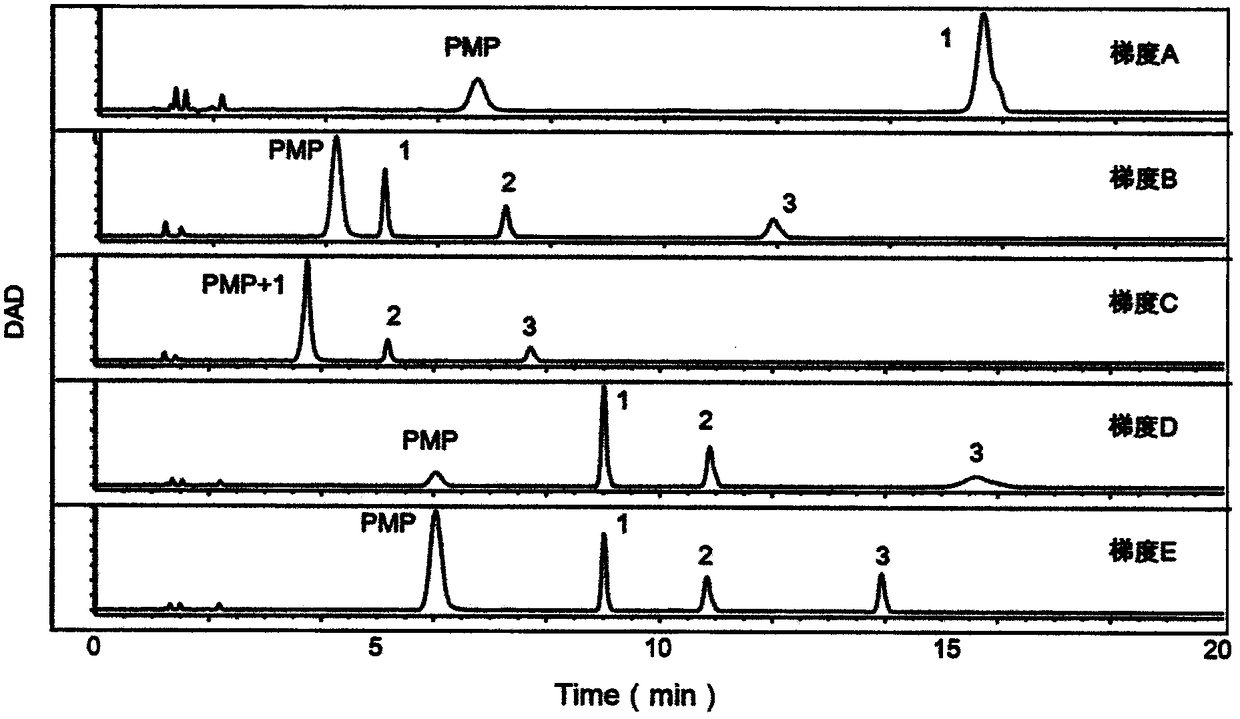
[0053] (1) Precisely weigh mannose (Man), glucosamine (GlcN), galactosamine (GalN), glucuronic acid (GlcUA), glucose (Glc), galactose (Gal), xylose (Xyl), rock Add appropriate amount of algalose (Fuc), add deionized water to prepare two mixed standard solutions containing the above monosaccharide 0.1mg / mL, and prepare immediately for use;
[0054] (2) Create an alkaline environment: Take 40 μL of the mixed monosaccharide standard in a 1.5mL EP tube, add 40 μL of 0.3mol / L sodium hydroxide, and vortex to mix;
[0055] (3) PMP derivatization: Add 60 μL 0.5mol / L 1-phenyl-5-methylpyrazolone (PMP) to each sample, vortex and mix well, and react in an oven at 70°C for 1 h after centrifugation;
[0056](4) Neutralization reaction by adding acid: Take out the samples in the oven, let them cool down, add 40 μL of 0.3mol / L hydrochloric acid to each sample, and vortex to mix;
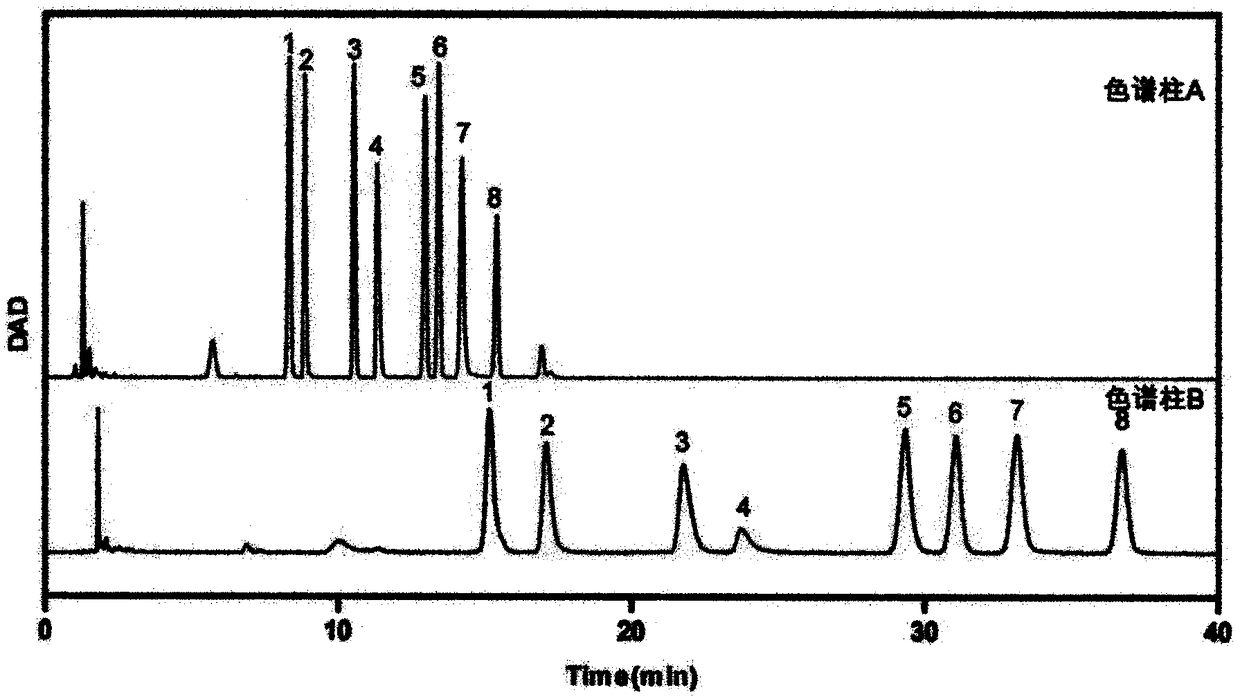
[0057] (5) Extraction: add 500 μL chloroform to each tube, vortex, centrifuge, remove the lower layer of chlorof...
Embodiment 2
[0082] (1) Accurately weigh the appropriate amount of mannose (Man), rhamnose (Rha) and glucose (Glc), add deionized water to prepare 5 parts of the same mixed standard solution containing the above monosaccharide 0.1mg / mL, and use it immediately match;
[0083] (2) Create an alkaline environment: Take 40 μL of the mixed monosaccharide standard in a 1.5mL EP tube, add 40 μL of 0.3mol / L sodium hydroxide, and vortex to mix;
[0084] (3) PMP derivatization: Add 60 μL 0.5mol / L PMP to each sample, vortex and mix well, and react in an oven at 70°C for 1 h after centrifugation;
[0085] (4) Neutralization reaction by adding acid: Take out the samples in the oven, let them cool down, add 40 μL of 0.3mol / L hydrochloric acid to each sample, and vortex to mix;
[0086] (5) Extraction: add 500 μL chloroform to each tube, vortex, centrifuge, remove the lower layer of chloroform, keep the supernatant, repeat 3 times;
[0087] (6) Centrifuge the sample at 13000r / min for 10min, take 80μL su...
Embodiment 3
[0109] (1) Accurately weigh the appropriate amount of mannose and glucose, add deionized water to prepare the above monosaccharides containing 0.5mg / mL, 0.25mg / mL, 0.1mg / mL, 0.05mg / mL, 0.01mg / mL, 0.005mg / mL, 0.0025mg / mL, 0.001mg / mL, 0.0005mg / mL mixed standard solution, ready-to-use;
[0110] (2) Create an alkaline environment: Take 40 μL of the mixed monosaccharide standard in a 1.5mL EP tube, add 40 μL of 0.3mol / L sodium hydroxide, and vortex to mix;
[0111] (3) PMP derivatization: Add 60 μL 0.5mol / L PMP to each sample, vortex and mix well, and react in an oven at 70°C for 1 h after centrifugation;
[0112] (4) Neutralization reaction by adding acid: Take out the samples in the oven, let them cool down, add 40 μL of 0.3mol / L hydrochloric acid to each sample, and vortex to mix;
[0113] (5) Extraction: add 500 μL chloroform to each tube, vortex, centrifuge, remove the lower layer of chloroform, keep the supernatant, repeat 3 times;
[0114] (6) Centrifuge the sample at 130...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com