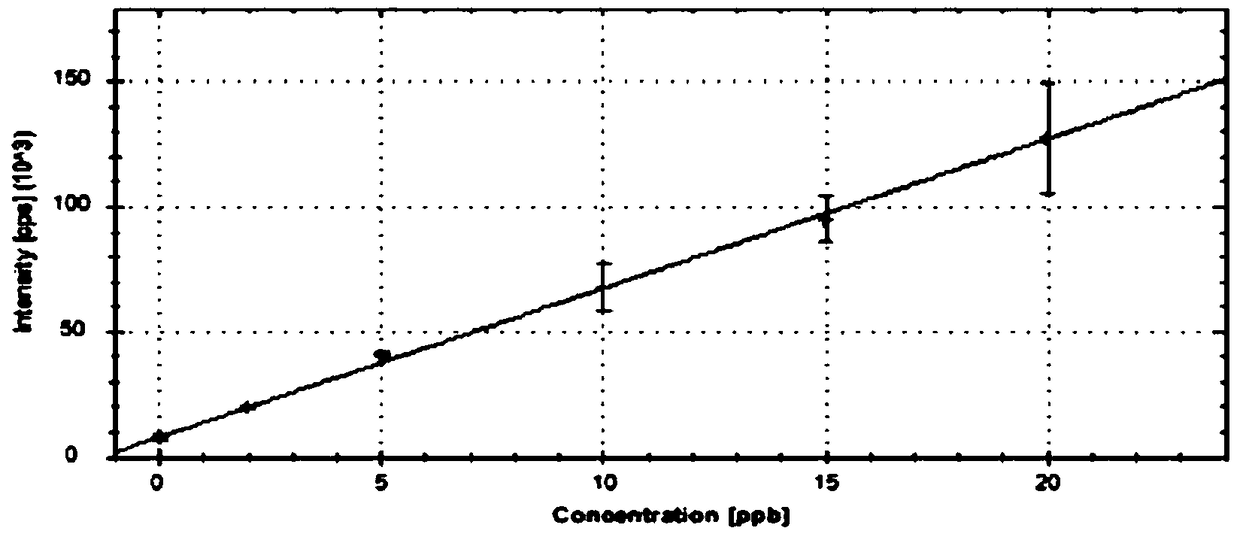
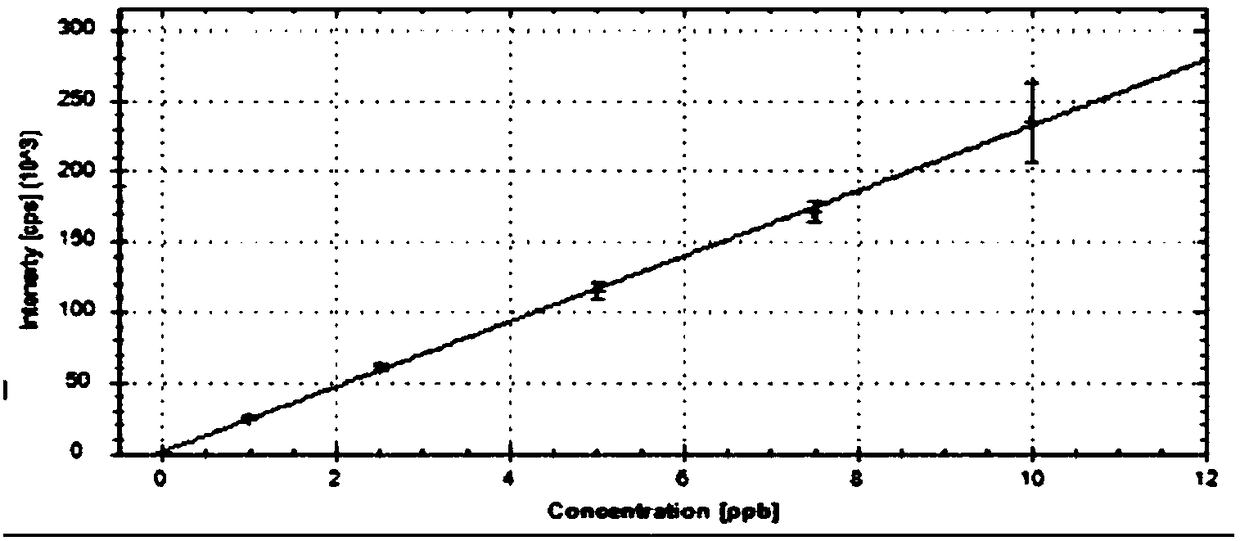
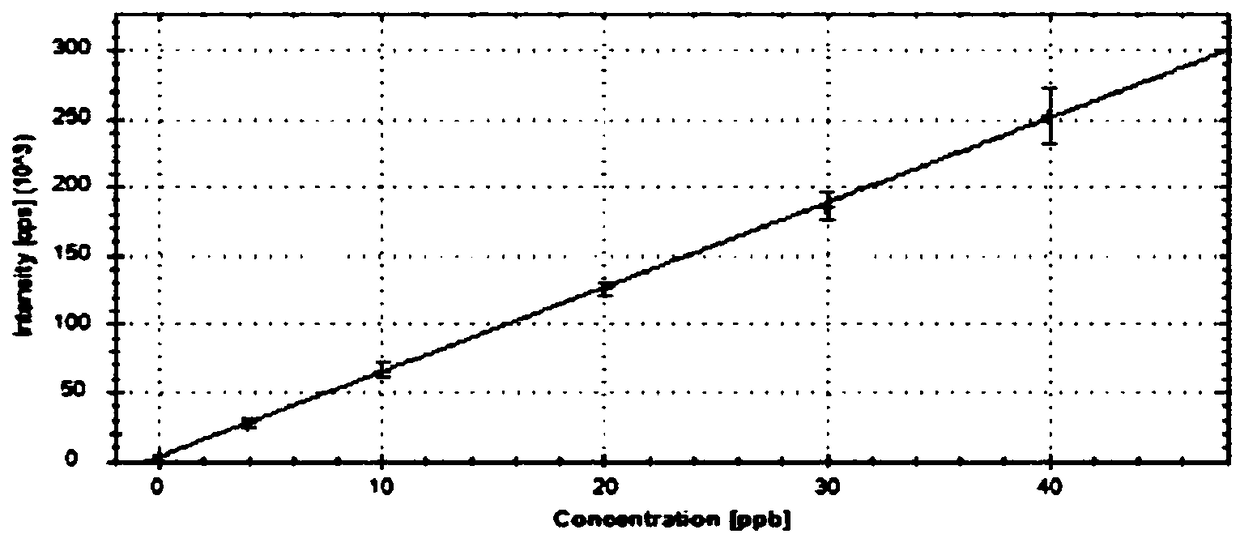
Method for determining content of 12 residual metals in medicines
A technology for residual metals and drugs, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effect of improving detection efficiency, good repeatability, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 - Obtainment of standard curve
[0064] Step 1: Preparation of the base solution
[0065] 1) Preparation of stock solution-1 (V: 50000ng / mL, Co: 25000ng / mL, Ni: 100000ng / mL, As: 75000ng / mL, Cd: 10000ng / mL, Hg: 15000ng / mL, Pb: 25000ng / mL) : Precisely pipet 0.50mL V standard solution, 0.25mL Co standard solution, 1.00mL Ni standard solution, 0.75mL As standard solution, 0.10mL Cd standard solution, 0.15mL Hg standard solution and 0.25mL Pb standard solution Transfer the standard solution to a 10mL volumetric flask, dilute to volume with 5% HNO3-2% HCl solution, and mix well.
[0066] 2) Stock solution-2 (V: 500ng / mL, Co: 250ng / mL, Ni: 1000ng / mL, As: 750ng / mL, Cd: 100ng / mL, Hg: 150ng / mL, Pb: 250ng / mL): Precisely pipette 1.00 mL of stock solution-1 into a 100 mL volumetric flask, dilute to volume with 5% HNO3-2% HCl solution, and mix well.
[0067] 3) Stock solution-3 (Li: 12500ng / mL, Sb: 4500ng / mL, Cu: 15000ng / mL, Zn: 50000ng / mL, Al: 50000ng / mL): Precisely pip...
Embodiment 2
[0117] Example 2 - Specificity Assay
[0118] Precisely pipette an appropriate amount of the standard solution of V, Co, Ni, As, Cd, Hg, and Pb, and dilute it with 5% HNO3-2% HCl solution to make each 1mL containing 10.0ng V, 5.0ng Co, 20.0ng Ni, A solution of 15.0 ng As, 2.0 ng Cd, 3.0 ng Hg, 5.0 ng Pb as reference solution-1. Precisely weigh an appropriate amount of this product, add 5.mL of 5% HNO3-2% HCl solution, ultrasonicate for 5 minutes to completely dissolve the drug, and dilute with 5% HNO3-2% HCl solution to prepare a sample solution containing 10 mg per 1 mL. As the test solution-1.
[0119]Precisely pipette appropriate amount of Li, Al, Cu, Zn, Sb element standard solution, dilute with 5% HNO3-2% HCl solution to make a solution containing 25ng Li, 100ng Al, 30ng Cu, 100ng Zn, 9ng Sb per 1mL , as the reference solution-2. Accurately weigh an appropriate amount of this product, add 5.mL 5% HNO3-2% HCl solution, ultrasonicate for 5 minutes to completely dissolve ...
Embodiment 3
[0127] Embodiment 3-accuracy analysis
[0128] The sample addition recovery test that present embodiment provides method:
[0129] The accuracy of the method provided by the invention is analyzed by the method of sample addition and recovery.
[0130] Take 100 mg of the drug, accurately weighed, and make 11 parallel portions. Take 2 of them, measure the content of V, Co, Ni, As, Cd, Hg, Pb elements in the sample; take 3 of them, add 0.1mL stock solution-2 respectively, add 5% HNO3-HCl solution to dissolve, and constant volume and mix well, as 50% recovery rate solution 1; take 3 parts, add 0.2mL stock solution-2, add 5% HNO3-HCl solution to dissolve, constant volume and mix, as 100% recovery rate Solution 1: Take 3 parts of it, add 0.3mL stock solution-2 respectively, add 5% HNO3-HCl solution to dissolve, constant volume and mix, and use it as solution 1 with 150% recovery rate of standard addition.
[0131] Take 25 mg of the drug, accurately weighed, and make 11 parallel p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com