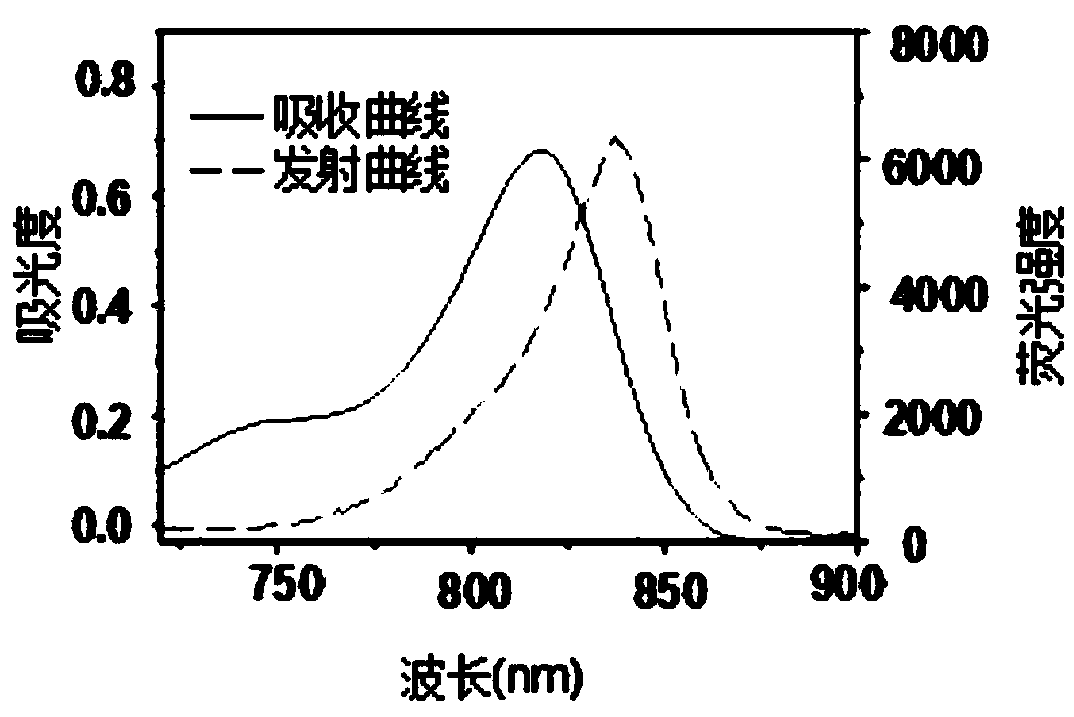
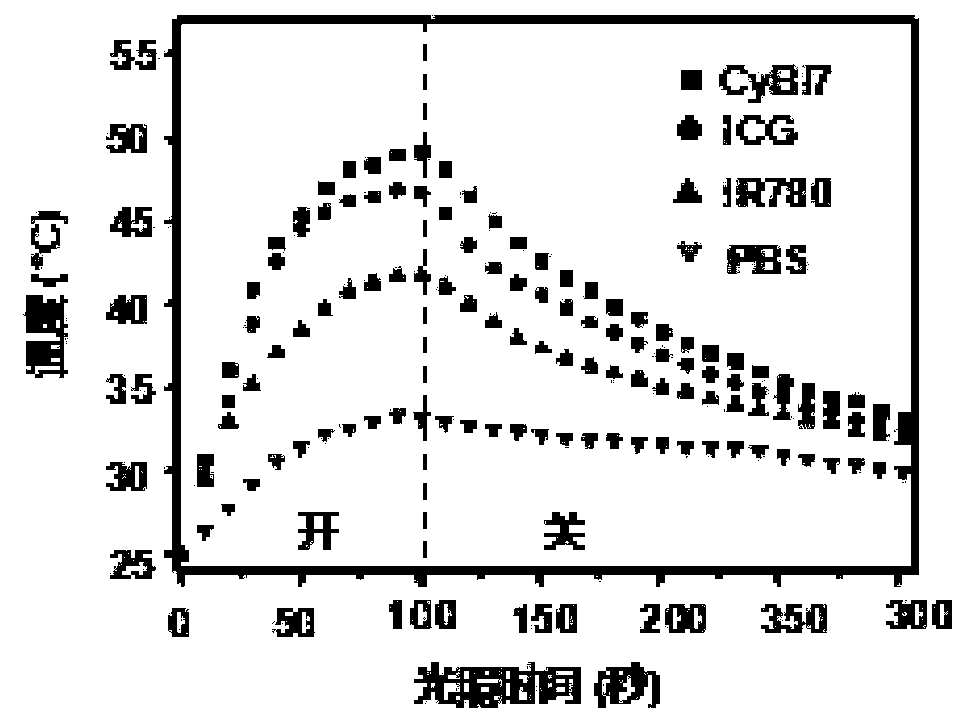
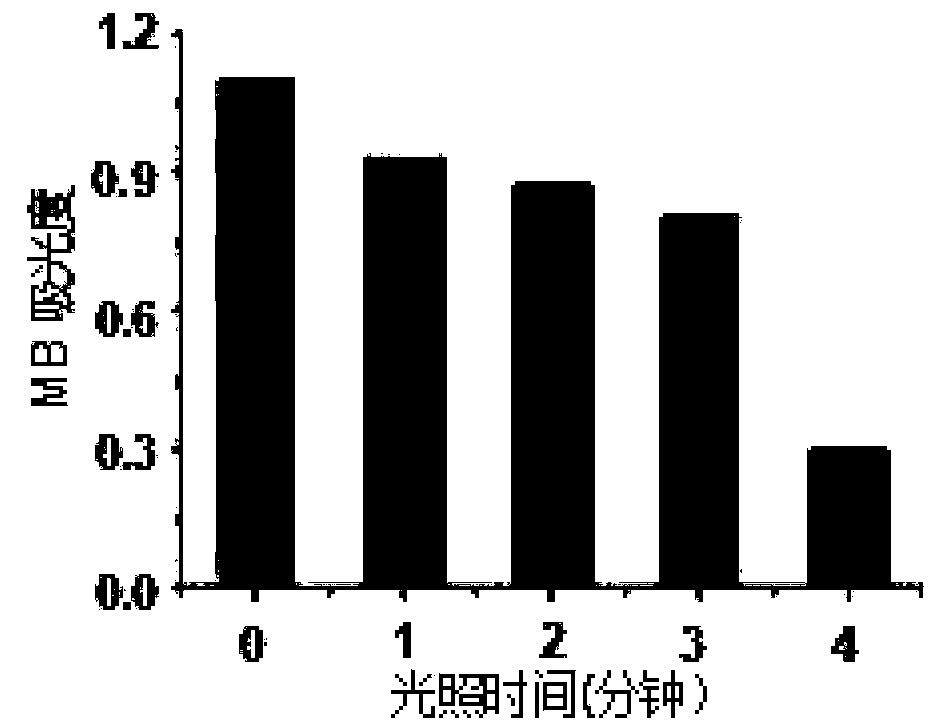
Conjugated chain functional benzoindole hematocyanine dye and application
A technology of benzoindole heptamethanine and benzindole heptamethanine, which can be used in tumor targeted imaging and treatment. The conjugated chain functionalized benzoindole heptamethanine dye field can Solve the problems of increased adverse conditions and side effects, probe waste, poor stability, etc., to achieve accurate and efficient photothermal and photodynamic anti-tumor, simplified synthesis steps, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0099] The synthesis method of the conjugated chain median functionalized benzindole heptamethine cyanine dye provided in the embodiment of the present invention comprises the following steps:
[0100] S101: In the mixed solution of DMF and CH2Cl2, add POCl3 dropwise, and then add cyclohexanone dropwise; the mixed solution is refluxed in a nitrogen atmosphere for 3 hours, and finally cooled to room temperature to obtain heptamethine with a chlorine substituent in the middle position Condensing agent A; wherein cyclohexanone: POCl 3 : The molar ratio of DMF is 1:5:15;
[0101] S102: React the benzindole quaternary ammonium salt B and the prepared heptamethine condensing agent A with chlorine substituents in glacial acetic acid and acetic anhydride for 1 to 2 hours to obtain a symmetry Heptamethine dye CyIC7; the molar ratio of indole quaternary ammonium salt B and pentamethine condensing agent A with chlorine substituents is 2:1;
[0102] S103: Combine the prepared compound C...
Embodiment 1
[0119] The preparation of Wujiachuan condensing agent compound A:
[0120]
[0121] A solution of 35 mL of dry dichloromethane and 37 mL of phosphorus oxychloride was dropped into a mixture of 80 mL of dry dichloromethane and N,N-dimethylformyl (1:1, v / v) under an ice bath. Keeping below 0°C, 10 g (0.10 mol) cyclohexanone was added dropwise to the reaction solution, and then the temperature was slowly raised to reflux for 3 h. Stop the reaction, cool in an ice-water bath, and pour the reaction solution into 200 g of crushed ice in batches. A large amount of red solid precipitated out. Filtrate under reduced pressure, wash the solid with frozen acetone in batches until yellow, and vacuum-dry to obtain a crude product, which is recrystallized with acetone to obtain 12.86 g of a yellow solid (Compound A), yield: 73%, melting point: 130-132°C.
Embodiment 2
[0123] Synthesis of dye CyBI7:
[0124]
[0125] Put 0.4g of benzindole quaternary ammonium salt B and 0.1g of heptamethine condensing agent C in 5mL of acetic anhydride, and reflux at 80°C for 1 to 2 hours. The molar ratio of feeding is strictly 2:1. When the molar ratio of feeding is less than 2:1, the by-product of pentamethine hemicyanine dye will be generated. Afterwards, the reaction solution was poured into 100 g of ice water to quench, and then 100 mL of CH was added to the ice water 2 Cl 2 , extraction, the organic layer was spin-dried under reduced pressure and separated by column chromatography (petroleum ether: dichloromethane, 1:20v / v), then the separated product solution was concentrated to 2mL, and then 20mL of diethyl ether was added to the concentrated solution to precipitate The solid was filtered to obtain 0.21 g of an earthy yellow dye with metallic luster. The NMR and mass spectrometry data of product structure identification are as follows:
[0126...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com