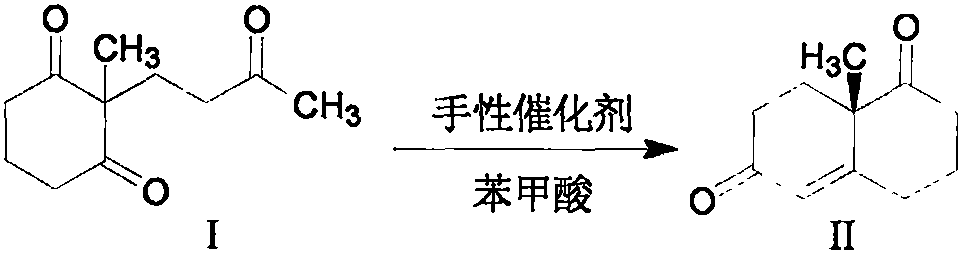
Preparation method of (S)-(+)-3,4,8,8a-tetrahydro-8a-methyl-1,6-(2H,7H)-naphthoquinone
A technology of methyl and naphthoquinone, which is applied in the field of -3, can solve the problems of discomfort, mediocre catalytic effect, and difficulty in catalyst synthesis, and achieve the effects of low dosage, excellent stereoselectivity, and excellent catalytic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] In a 100mL flask, 71.61mmol of I, 0.5mol% of L-prolinamide compound III and 1.79mmol of benzoic acid were mixed uniformly, stirred at 20°C for 4 days, and the system turned black. Afterwards, the reaction solution was transferred to a 250 mL round-bottomed flask with 100 mL of ethyl acetate, and the organic phase was washed with 30 mL of saturated sodium bicarbonate and 30 mL of saturated brine, respectively, and dried over anhydrous sodium sulfate. Afterwards, 3 g of activated carbon (100 mesh) was added to the organic phase, stirred at 20° C. for 15 hours, and filtered under normal pressure. The filtrate was precipitated under reduced pressure, and the residue was dissolved in 10 mL of methyl tert-butyl ether, frozen at -25°C for 24 hours, and filtered to obtain the precipitated brown solid, namely (S)-(+)-3,4,8,8a -Tetrahydro-8a-methyl-1,6-(2H,7H)-naphthoquinone (II), yield 86%, ee=99%.
example 2
[0024] In a 500mL flask, 572.88mmol (112.42g) of I, 0.5mol% of L-prolinamide compound III and 14.32mmol of benzoic acid were mixed uniformly, stirred at 20°C for 5 days, and the system turned black. Afterwards, the reaction solution was transferred to a 2000 mL round-bottomed flask with 800 mL of ethyl acetate, and the organic phase was washed with 240 mL of saturated sodium bicarbonate and 240 mL of saturated brine, respectively, and dried over anhydrous sodium sulfate. Afterwards, 24 g of activated carbon (100 mesh) was added to the organic phase, stirred at 20° C. for 15 hours, and filtered under normal pressure. The filtrate was precipitated under reduced pressure, and the residue was dissolved in 80 mL of methyl tert-butyl ether, frozen at -25°C for 24 hours, and filtered to obtain the precipitated brown solid, namely (S)-(+)-3,4,8,8a -Tetrahydro-8a-methyl-1,6-(2H,7H)-naphthoquinone (II), yield 82%, ee=99%.
example 3
[0026]In a 100mL flask, 71.61mmol of I, 1.0mol% of L-proline amide compound III and 1.79mmol of benzoic acid were mixed uniformly, and reacted for 4 hours under 5W microwave radiation conditions, and the temperature of the control system was not more than 45°C. The color becomes darker. Afterwards, the reaction solution was transferred to a 250 mL round-bottomed flask with 100 mL of ethyl acetate, and the organic phase was washed with 30 mL of saturated sodium bicarbonate and 30 mL of saturated brine, respectively, and dried over anhydrous sodium sulfate. Afterwards, 3 g of activated carbon (100 mesh) was added to the organic phase, stirred at 20° C. for 15 hours, and filtered under normal pressure. The filtrate was precipitated under reduced pressure, and the residue was dissolved in 10 mL of methyl tert-butyl ether, frozen at -25°C for 24 hours, and filtered to obtain the precipitated brown solid, namely (S)-(+)-3,4,8,8a -Tetrahydro-8a-methyl-1,6-(2H,7H)-naphthoquinone (II)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com