A kind of zl006 cyclohexyl ester polymer nano drug delivery system and preparation method thereof
A nano drug delivery system, cyclohexyl ester technology, applied in the field of ZL006 cyclohexyl ester polymer nanocarrier and its preparation, can solve the problem of poor solubility of ZL006 cyclohexyl ester, reduce off-target distribution and toxicity, and the method is simple and easy , good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
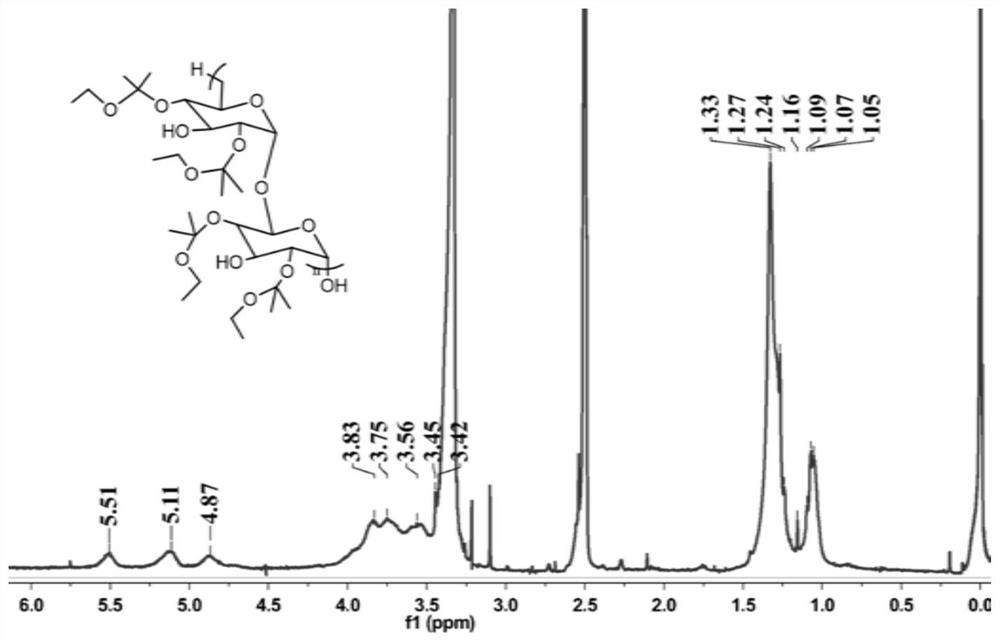
[0025] Embodiment 1: Preparation and characterization of carrier material m-Dextran
[0026] Take 1.0 g of dextran (Mn ~ 9-11kDa) into a dry round bottom flask, and blow dry with nitrogen. Add 10 mL of anhydrous dimethyl sulfoxide and stir until the dextran is fully dissolved. 4.16 mL (37 mmoL) of ethoxypropylene and 15.6 mg (0.062 mmoL) of pyridinium p-toluenesulfonate (PPTS) were added successively. Nitrogen was passed through the reaction solution, and after blowing for 2-3 minutes, it was sealed with a parafilm to prevent the solvent from volatilizing. Stir at room temperature for 30 min to obtain m-Dextran, and then add 1 mL of triethylamine to terminate the reaction. The reaction resulted in a white precipitate, which was washed three times with alkaline aqueous solution (pH ~ 8) to prevent degradation, and the product was purified by high-speed centrifugation (8000 rpm, 15 min). Lyophilization to remove excess water gave the product (m-Dextran) as a white solid. Nuc...
Embodiment 2
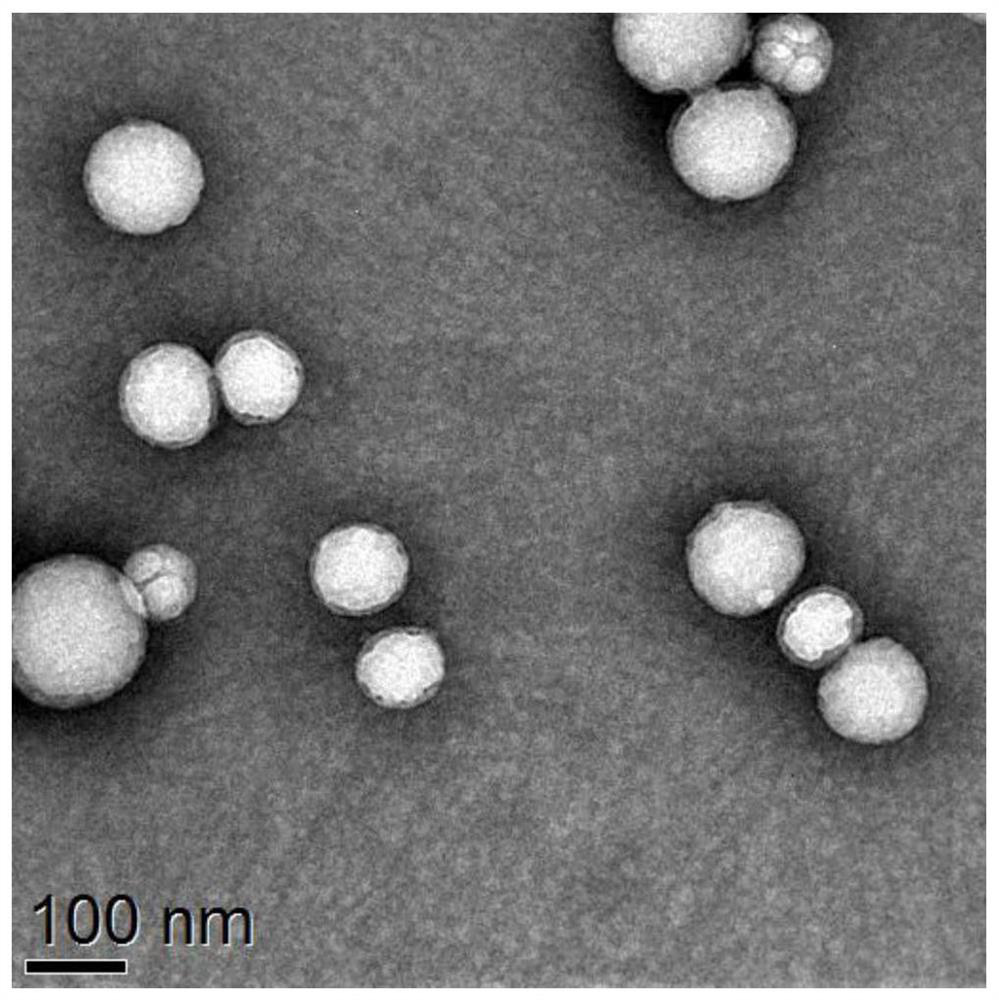
[0027] Example 2: Preparation and Characterization of ZL006 Cyclohexyl Ester Polymer Nano Drug Delivery System
[0028] Take 4 mg of neuroprotective agent ZL006 cyclohexyl ester and 20 mg of m-Dextran prepared in Example 1 in a 15 mL centrifuge tube, add 2 mL of dichloromethane, vortex until completely dissolved, add 4 mL of PVA solution prepared by 3% PBS (pH7.4) . Ultrasonic cell disruptor ultrasonication in ice bath (35% power, ultrasonic 2s / stop 2s, 5min). The white emulsion after sonication was slowly introduced into 15 mL of PVA solution prepared with 0.3% PBS (pH 7.4) in high-speed stirring (800 rpm). After continuous stirring for about 1-2h, the dichloromethane was completely volatilized, and the solution was clear and transparent, showing blue nanoparticle opalescence. Centrifuge at high speed (12000rpm, 40min), discard the supernatant, add water to redissolve, repeat 2-3 times to wash away the emulsifier, and finally disperse in 1mL deionized water to obtain a poly...
Embodiment 3
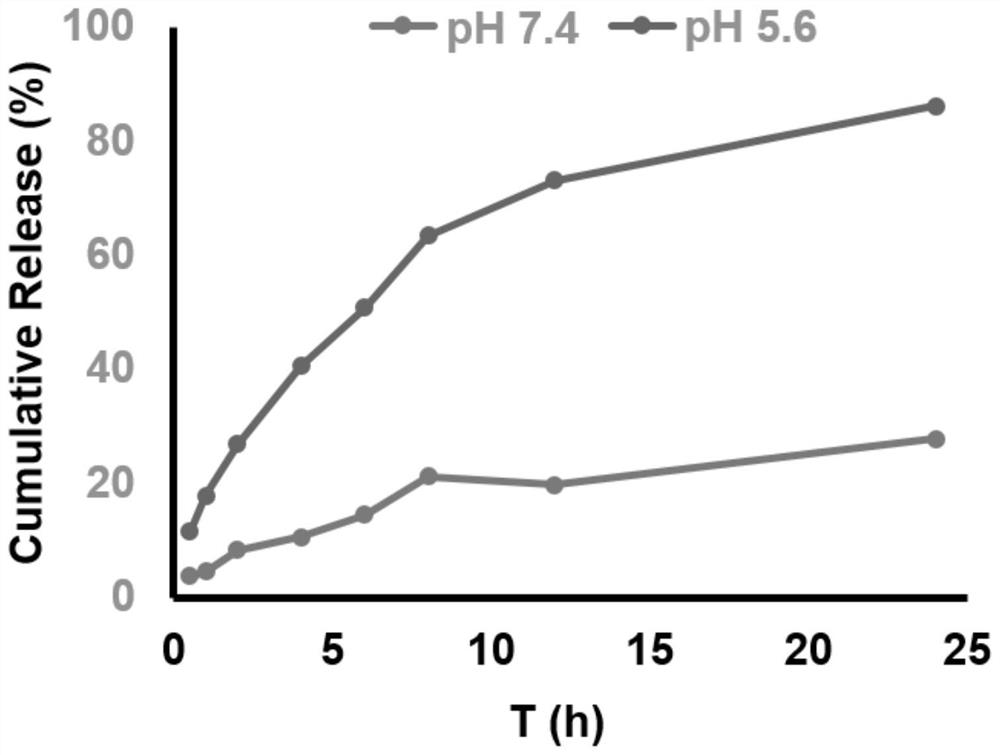
[0029] Example 3: Determination of Encapsulation Efficiency (EE%) and Drug Loading Capacity (DL%) of ZL006 Cyclohexyl Ester Polymer Nano-Drug Delivery System
[0030] (1) HPLC method to establish ZL006 standard curve:
[0031] Chromatographic conditions, column: HanbonPhecda C18 (4.6mm×150mm, 5μm; Jiangsu Hanbon Technology Co., Ltd.); mobile phase: methanol-water (90:10; v / v); flow rate: 1.0mL / min; UV detection wavelength : 311nm; column temperature: 30°C; injection volume 20μL.
[0032] Standard curve drawing: Accurately weigh 0.0252 g of ZL006 cyclohexyl ester dried at 105°C to constant weight in a 50 mL volumetric flask, dilute to the mark with mobile phase, and obtain a standard stock solution with a concentration of about 504 μg / mL. Precisely pipette 0.05, 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, 8.0, 10.0mL of a series of stock solution into a 50mL volumetric flask, and dilute the mobile phase to the mark to obtain 1.0, 2.0, 5.0, 10.0, 20.0, 50.0 , 80.0, and 100.0 μg / mL sample solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com