Fluorescent immunochromatographic reagent kit for detecting human helicobacter pylori antibody and preparation method of reagent kit
A technology of Helicobacter pylori and fluorescence immunochromatography, applied in biological testing, fluorescence/phosphorescence, material analysis by optical means, etc., can solve the problems of long time, complicated preparation method, high price, etc. Simple, fast and low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
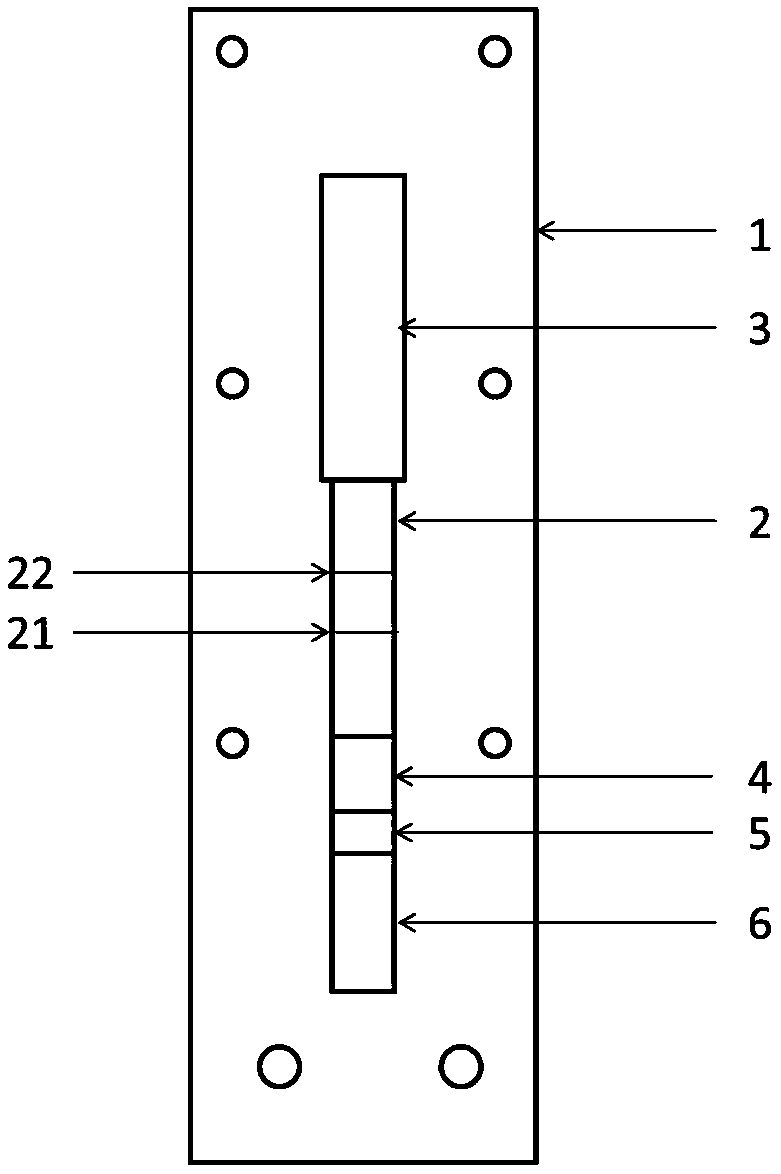
[0047] The present embodiment Helicobacter pylori detection kit, such as figure 1 As shown, it includes a support 1, an imprinted membrane 2 arranged on the support 1, an absorbent pad 3 arranged at one end of the imprinted membrane 2, a binding pad 4 at the corresponding other end, and a sample pad 5 arranged at the other end of the binding pad 4, Set on the other end of the sample pad 5 is a blood filter membrane 6 . Wherein, the support 1 is made of a plastic plate, and the imprinted membrane 2 is made of a nitrocellulose membrane. A detection line 21 and a control line 22 are set on the nitrocellulose membrane, the detection line 21 is coated with Caga A, urease and vacA recombinant antigen mixture; the control line 22 is coated with goat anti-mouse IgG. The binding pad 4 is sprayed with fluorescent latex microspheres coupled with mouse anti-human IgG. The mixed mass ratio of Caga A, urease and vacA recombinant antigen coated on the nitrocellulose membrane detection line...
Embodiment 2
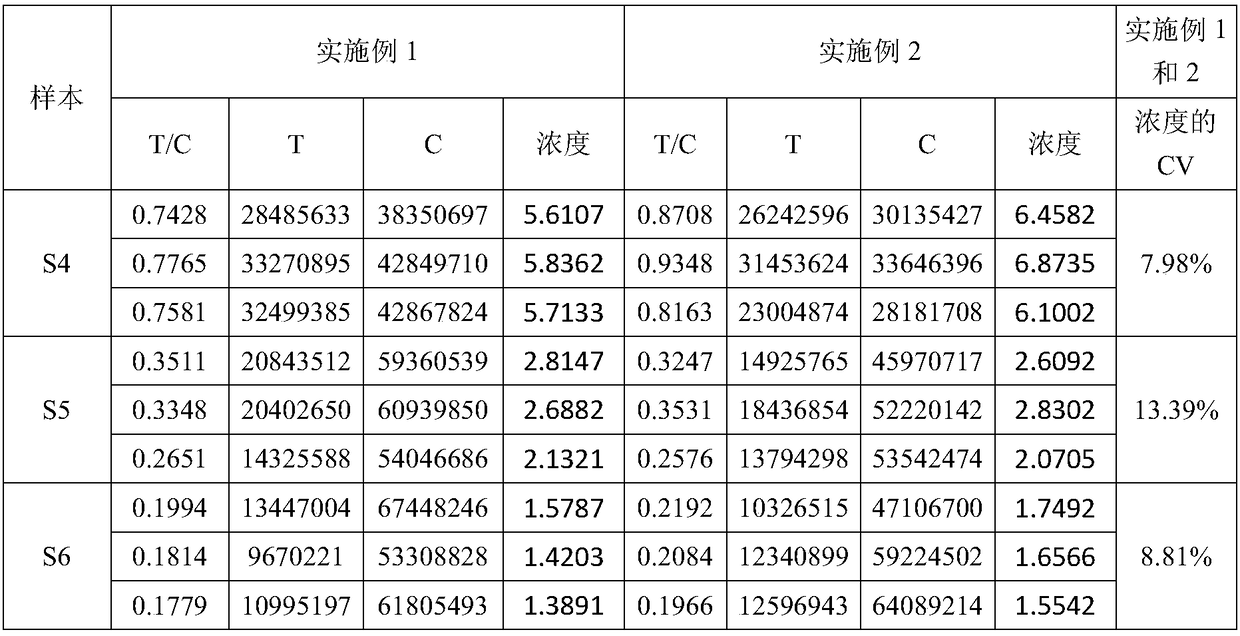
[0072] The difference between the Helicobacter pylori detection kit in this example and Example 1 is that during the process of coupling the microspheres to the antibody, the microspheres were coupled on a shaking table at 100-150 rpm at 37°C for 1 hour, and 500uL was directly added for blocking after coupling without centrifugation. The active groups of the microspheres were blocked with liquid, and blocked on a shaker at 150 rpm at 37°C for 30 min. Others are the same as embodiment 1. Embodiment 1 and embodiment 2 detect the same sample, and the data are shown in Table 1.
[0073] Table 1
[0074]
[0075]
[0076] The coefficient of variation CV of the detected concentration is less than 15% when the same sample is detected in the embodiment 1 and the embodiment 2, and there is no significant difference in the detection results of the two reagent strips in the embodiment 1 and the embodiment 2.
Embodiment 3
[0078] The Helicobacter pylori antibody was diluted into 6 concentration gradients of 1.56ug / L, 3.13ug / L, 6.25ug / L, 12.5ug / L, 25ug / L, and 50ug / L as the standard solution, and a blank solution was prepared at the same time.
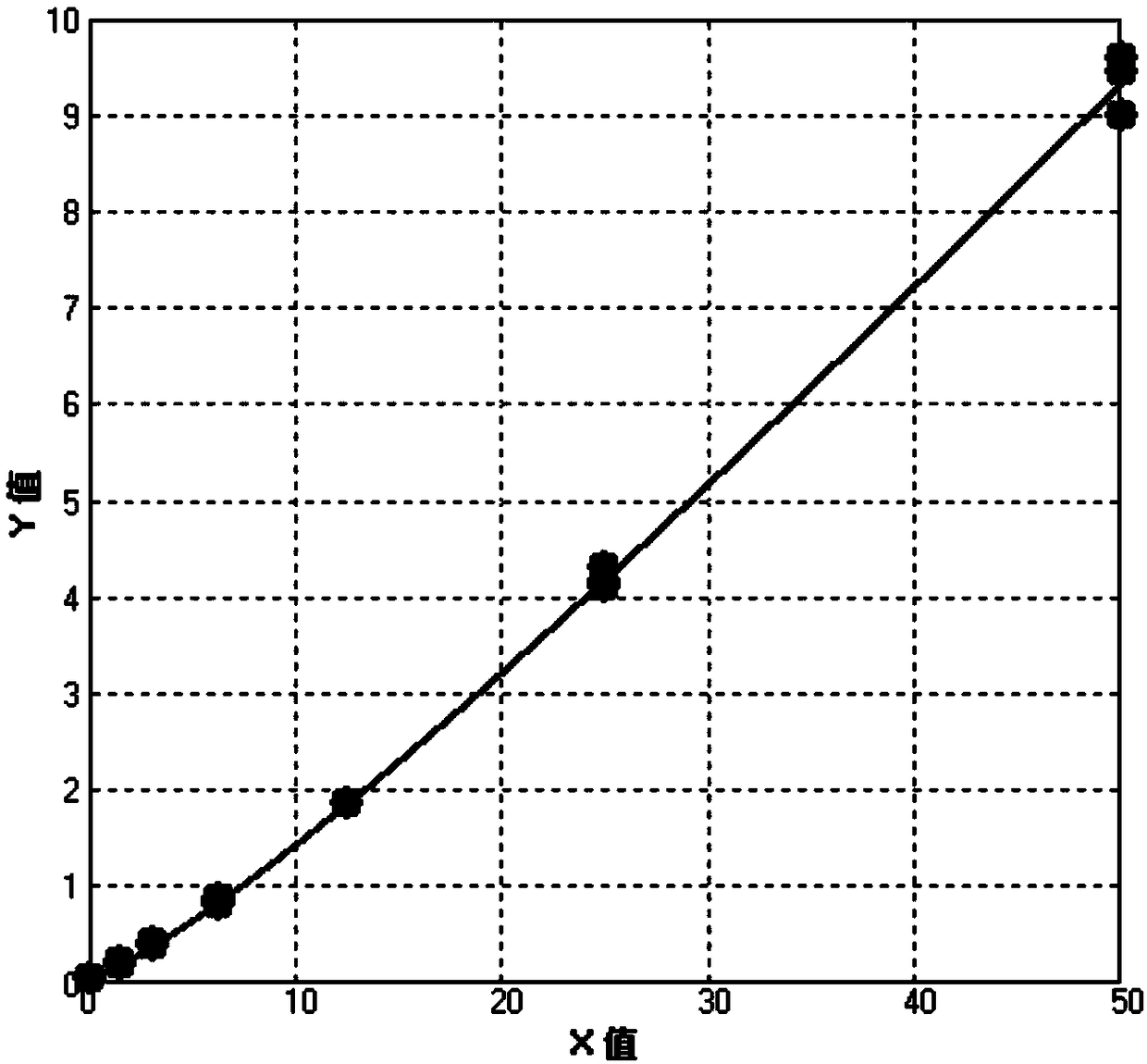
[0079] Take 80 microliters each of 6 standard products and blank solutions and add them to the sample area (sample pad) of the reagent strip, and insert them into the HitFia-Biohan Fluorescence Immunoassay Analyzer after 15 minutes (Biohan Biotechnology (Hefei) Co., Ltd. ) readings, each standard was detected 3 times. Read the luminescence value and T / C value of the detection line (T line) and control line (C line), see Table 2. Calculate the relationship between the T / C value and the concentration of the corresponding standard substance, draw the standard curve, see figure 2 . The standard curve regression equation is shown in Table 3. The detection limit can reach 0.5ug / L.
[0080] Table 2
[0081]
[0082] Table 3 Four-parameter Logistic curve ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com