Nucleic acid releasing agent and nucleic acid on-site releasing method
A technology of nucleic acid release agent and liquid preparation, which is applied in the fields of biochemical equipment and methods, DNA preparation, microbial measurement/inspection, etc. It can solve the problems of long sample processing time, long time, and not widely used, so as to solve the loss of nucleic acid and pollution, low manufacturing cost and safe use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention also provides a preparation method of a nucleic acid rapid release agent, comprising the following steps: mixing nonionic surfactant, potassium chloride, guanidine isothiocyanate, bovine serum albumin, ethylenediaminetetraacetic acid, trehalose, Tris base, proteinase K and water are mixed to prepare a solution, the pH value is adjusted to 7.2-7.7 with HCl, and the solution is sterilized by filtration to obtain the nucleic acid releasing agent. The preparation method of the nucleic acid releasing agent in the present invention is simple and meets the requirement of convenient detection.
[0037] The present invention also provides a nucleic acid releasing agent on-site nucleic acid release method, comprising the following steps: 1) mixing the nucleic acid releasing agent with a sample to obtain a mixed solution; 2) leaving the mixed solution in step 1) for 3- After 10 minutes, the solid-liquid separation was performed, and the solid-phase components...
Embodiment 1
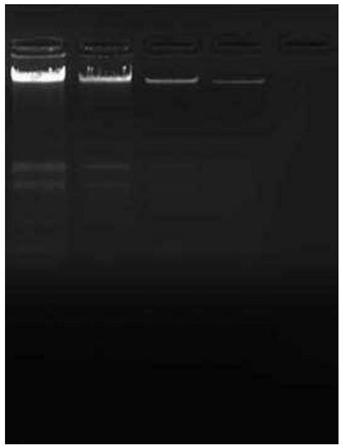
[0043] The method of the present invention, the boiling method, the centrifugal column extraction method, and the magnetic bead extraction method are respectively used to extract DNA from a Salmonella culture, measure the OD value of nucleic acid, and perform electrophoresis detection at the same time.
[0044] The proportion of each component of the nucleic acid releasing agent described in the present embodiment is: the nonionic surfactant of 0.6% by volume, the potassium chloride of 100mM, the guanidine isothiocyanate of 20mM, the bovine serum albumin of 1.5mM, 3mM EDTA, 0.25% by mass volume trehalose, 3mg / ml proteinase K, 1.5mM Tris.HCl (PH7.5), and sterile water as the balance. Extraction steps: draw 10 μL of nucleic acid release agent and 20 μL of Salmonella culture sample respectively, put them into a 0.5mL Eppendorf centrifuge tube, repeatedly blow and beat several times with a pipette, mix well, then let the mixed solution stand for 5min, and centrifuge at 2000rpm to o...
Embodiment 2
[0051] The proportion of each component of the nucleic acid release agent described in the present embodiment is: 0.3% by volume of non-ionic surfactant, 80mM potassium chloride, 20mM guanidine isothiocyanate, 2mM bovine serum albumin, 5mM EDTA, 0.5% trehalose by mass volume, 0.5 mg / ml proteinase K, 1 mM Tris.HCl (pH 7.5), and sterile water as the balance. Extraction steps: draw 5 μL of nucleic acid release agent and 5 μL of hepatitis B virus sample respectively, put them into a 0.5mL Eppendorf centrifuge tube, repeatedly blow and beat several times with a pipette, mix well, then let the mixed solution stand for 5min, and centrifuge at 2000rpm to obtain the target DNA nucleic acid.
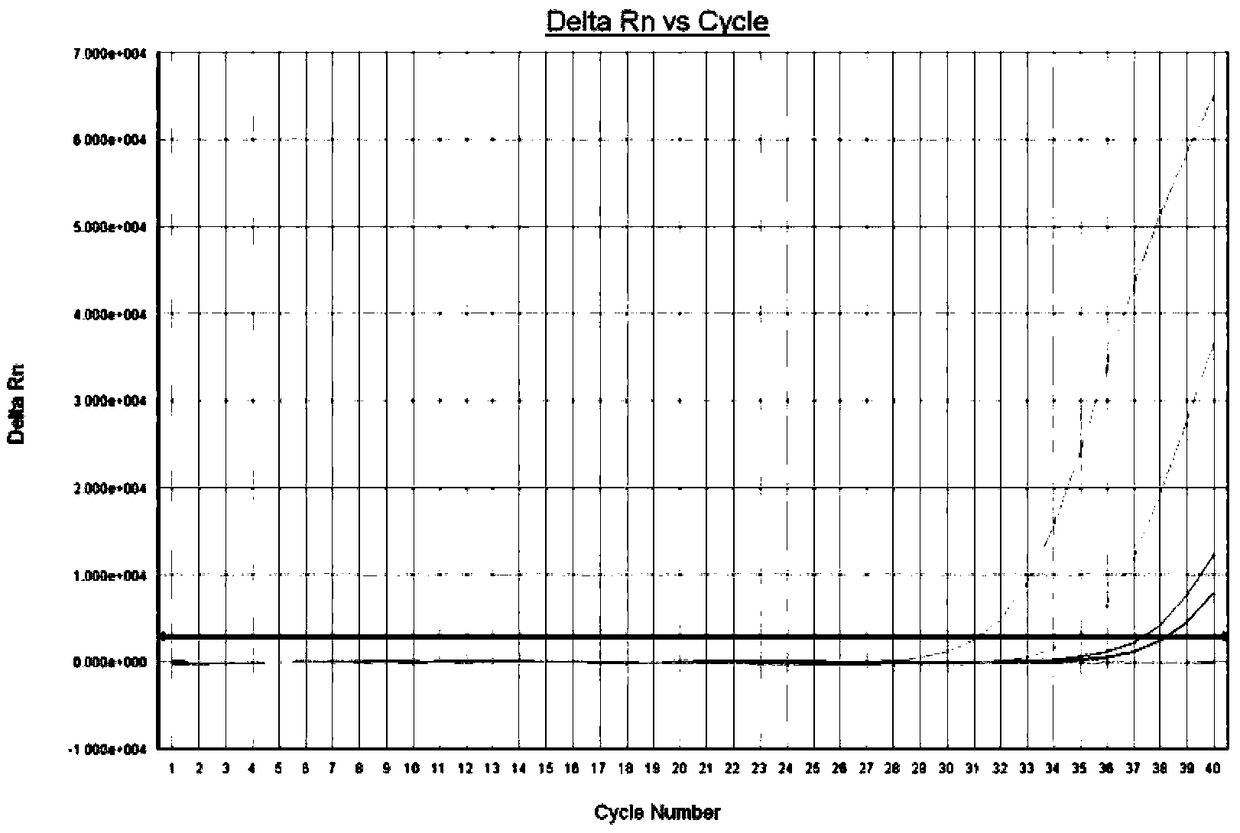
[0052] In this embodiment, nucleic acid release agents were used to extract 4 cases of hepatitis B virus positive serum samples. After nucleic acid extraction, 2 μL of nucleic acid extraction solution was respectively drawn, and added to the hepatitis B virus fluorescent PCR commercial detection k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com