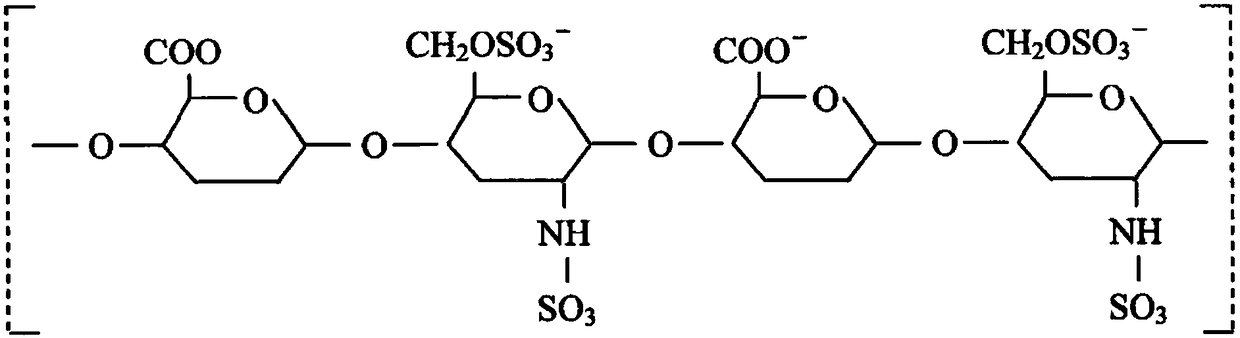
Preparation method of heparin sodium
A technology of heparin sodium and pig small intestine, applied in the field of biochemical industry, can solve the problem of not being able to release heparin sodium, and achieve the effect of solving the problem of poisoning and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The purpose of the present invention is to solve the problem that simple salt hydrolysis and simple enzymatic hydrolysis cannot completely release heparin sodium from intestinal mucosal cells, that is, the combined process of salt hydrolysis and enzymatic hydrolysis can completely dissociate heparin from intestinal mucosal cells, thereby completely Extraction problem. And found more objective production parameters for industrial scale production.
[0027] Concrete steps of the present invention are as follows:
[0028] Autoclave all equipment, reagents, and consumables before the test;
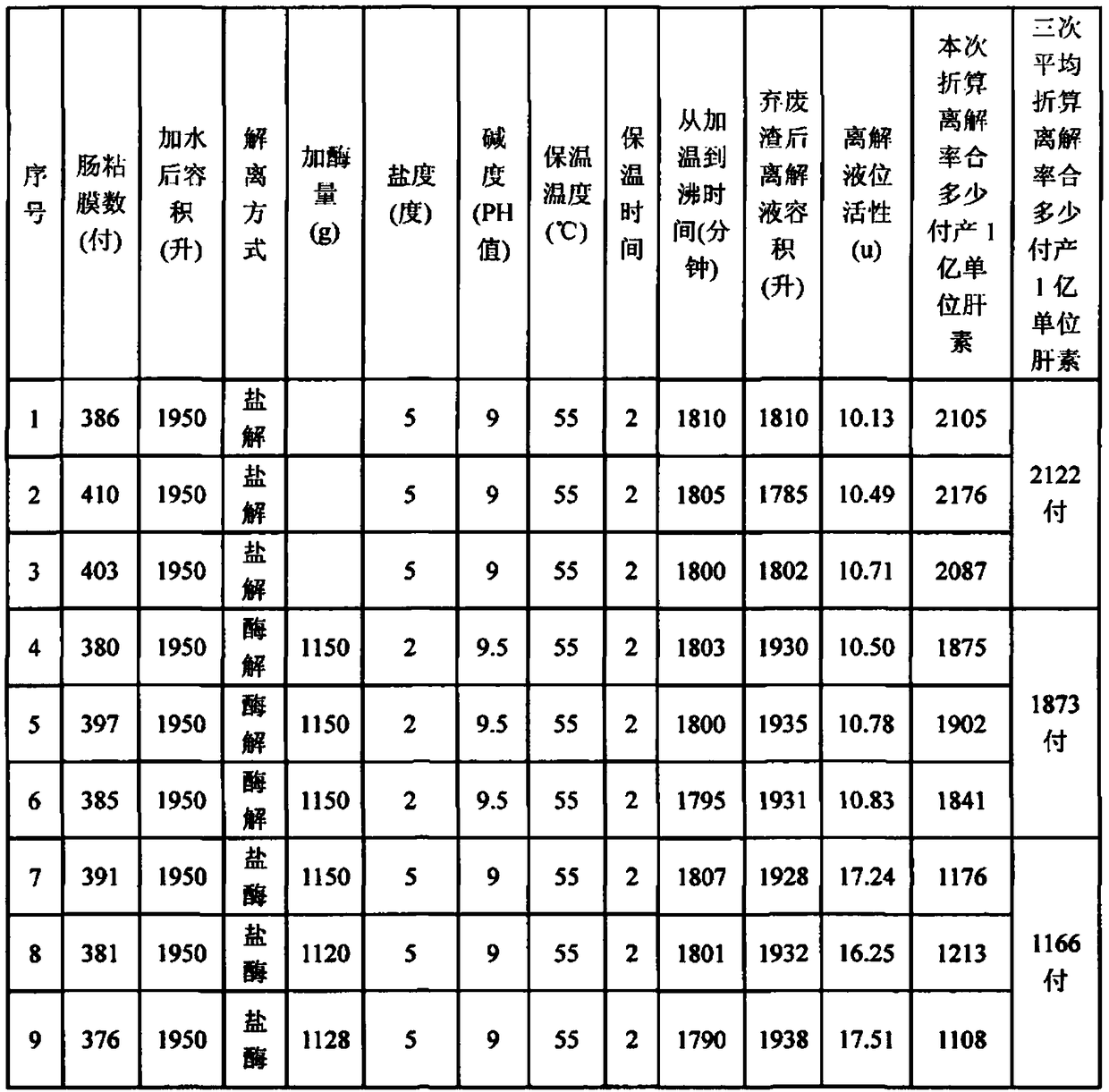
[0029] 1) Dissociation: According to the weight ratio of water: pig small intestine mucosa is 4~5: 1, add water to the pig small intestine mucosa, and add salt to make the liquid salinity 5 degrees, add NaOH to adjust the pH value to 9, stir and heat up to 55°C, after 30 minutes of heat preservation, add 0.5g of alkaline protease 2709 per kilogram of porcine small intestinal mucosa to...
Embodiment 2
[0041] (1), in a 2000-liter reaction tank, pump 400 pairs of pig small intestinal mucosa and water to occupy 90% of the space, add 120 kg of salt, add alkali to adjust the pH value to 9, heat up to 55 ° C, and keep warm for 30 minutes. Alkaline protease (type 2709) 1000 grams, after 2 hours of heat preservation, continue to heat up to 85 ℃, filter and cool to about 55 ℃.
[0042] (2) Add 8000 grams of basic resin Type 98 to the liquid at 55° C., stir and absorb for 8 hours, and filter to wash the resin.
[0043] (3) Add the same amount of 15°C saline as the resin, and perform elution twice, each time for 1 hour, and mix the above-mentioned elution water together.
[0044] (4) Add industrial alcohol to the upper eluent to make the alcohol concentration 45 degrees, let it stand for 24 hours, filter the lower sediment with polyester cloth, and dry it in a hot water bath at 65-75 ° C to obtain a purity of 90 The above activity units / mg heparin sodium product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com