Use of compound AG120 or pharmaceutically acceptable salt thereof in preparation of drug for prevention or treatment of tuberculosis
A technology of AG120 and compound, applied in the direction of antibacterial drugs, dipeptide components, etc., can solve the problem of the use of unreported compound AG120 anti-tuberculosis drugs, and achieve the effect of effective drug support and inhibition of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
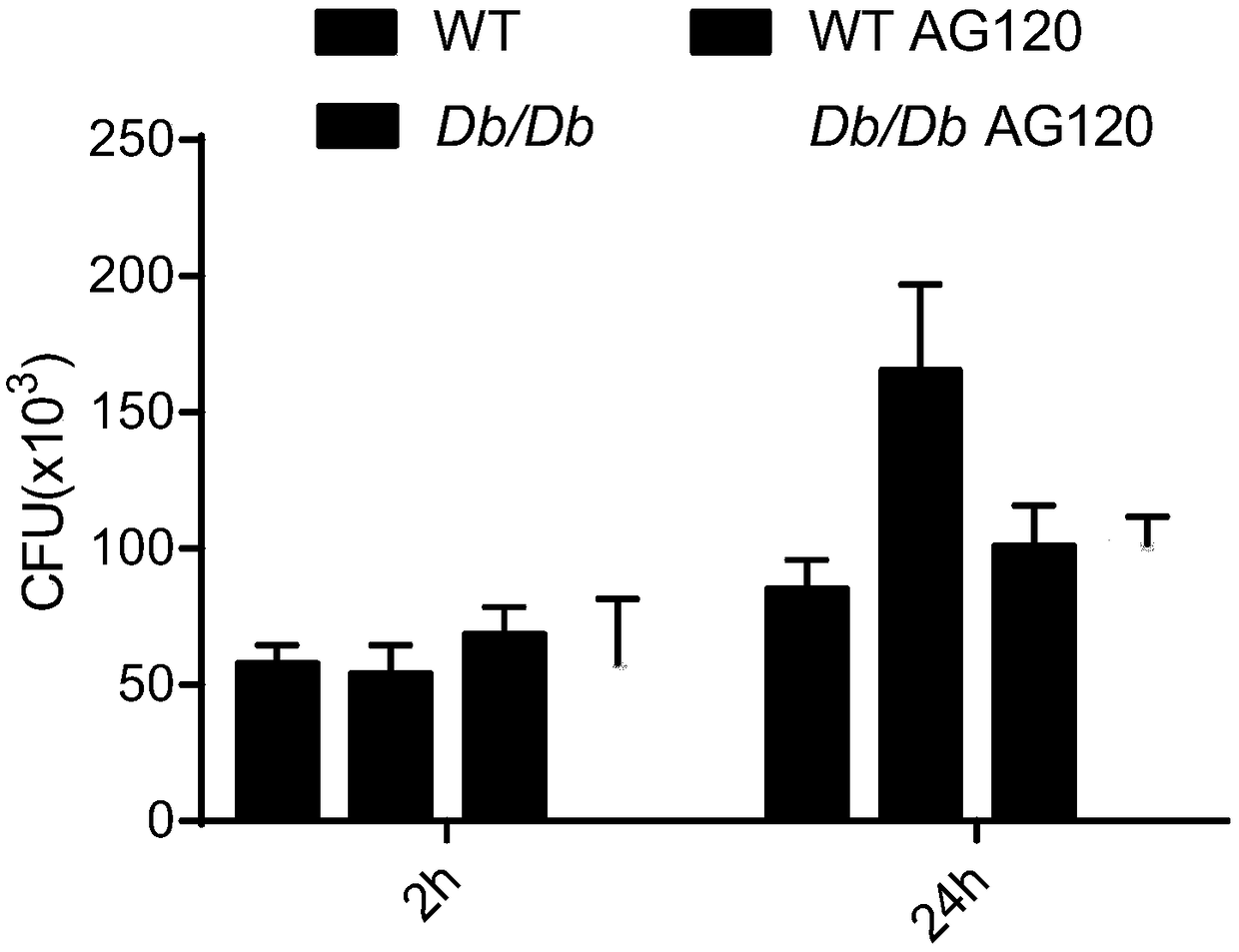
[0030] Db / Db (leptin receptor (Lepr) mutant) peritoneal primary macrophages of C57BL / 6 mice and DB / db mice were mixed with 1*10 5 Each cell / well was inoculated in a 48-well cell culture plate. After about 2 hours for the cells to adhere to the wall, the complete medium 1640 was removed, and then fresh complete medium was added to culture overnight. On the next day, 1 hour before infection, fresh 1640 containing 10% serum without double antibody was used to infect Mycobacterium tuberculosis (H37Rv) at a dose of MOI=5. After 2-3 hours of infection, discard the supernatant, then culture the cells with amikacin-containing medium for 2 hours, discard the supernatant and replace with 1640 containing 10% serum without double antibody and continue at 37°C for 5 %CO 2 Cells were cultured in the incubator for 24 h. Discard the supernatant and wash the cells with PBS, then lyse the cells with PBS containing 1% triton-100, take the cell lysate and spread it on a MiddleBook 7H10 agar pla...
Embodiment 2
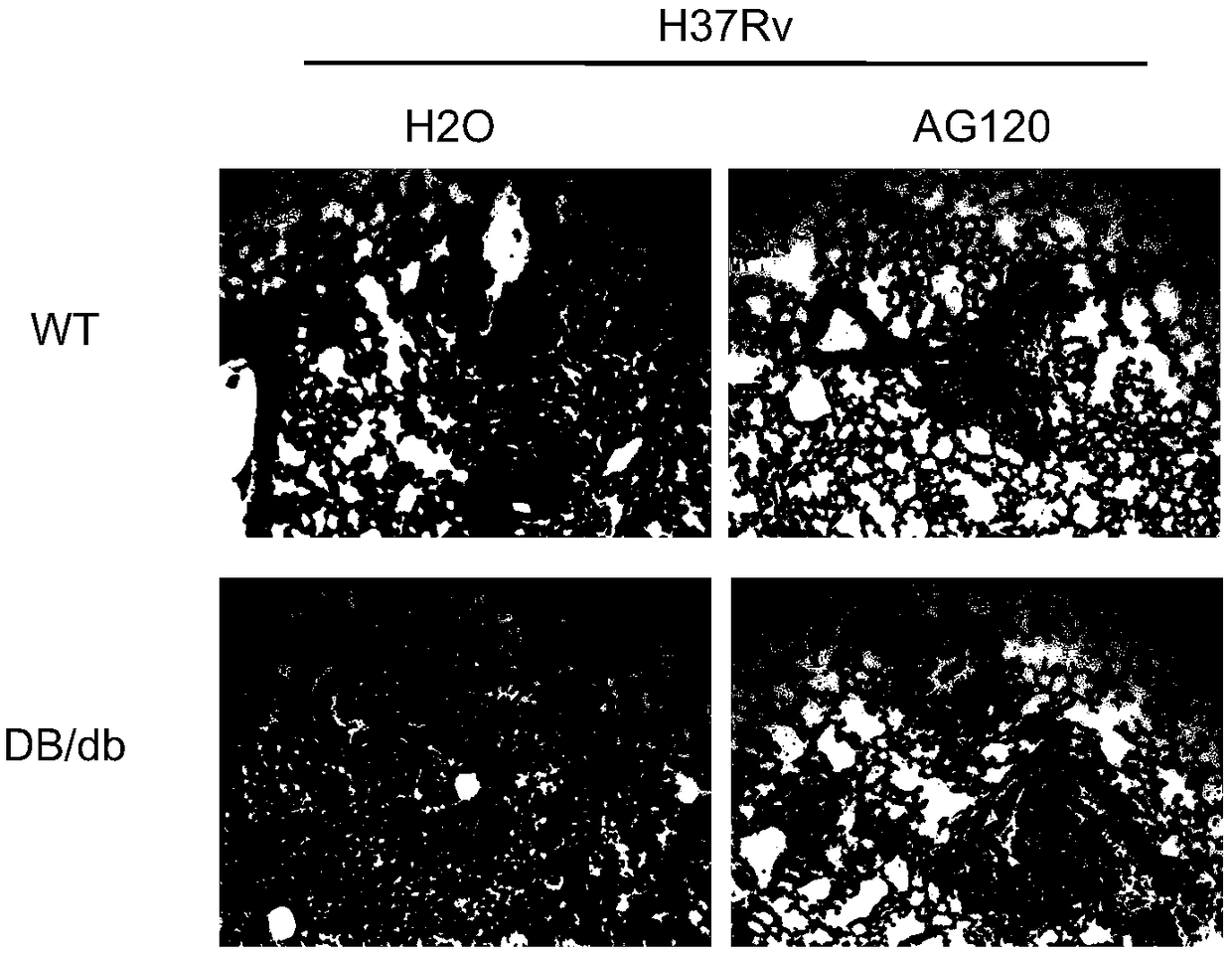
[0033] The C57BL / 6 mice were divided into two groups, and the Db / db mice were divided into two groups, with 6 mice in each group, which were infected with Mycobacterium tuberculosis (H37Rv) by intranasal drip at a dose of 200 CFU / mouse for 1 week. Afterwards, the compound AG120 was orally administered continuously for 3 weeks at a dose of 2 mg / kg / day, and pure water was used as a blank control. The mice were sacrificed by dislocation of the cervical spine, and one lobe of the lung was taken out, fixed with 4% paraformaldehyde, paraffin sectioned, and H&E staining was performed to observe the pathological damage of the lung.
[0034] Experimental results such as figure 2 As shown, the pathological damage to the lungs of the mice treated with the compound AG120 was significantly reduced; therefore, the experimental results in this example show that the compound AG120 can effectively reduce the pathological damage to the lungs of the mice.
Embodiment 3
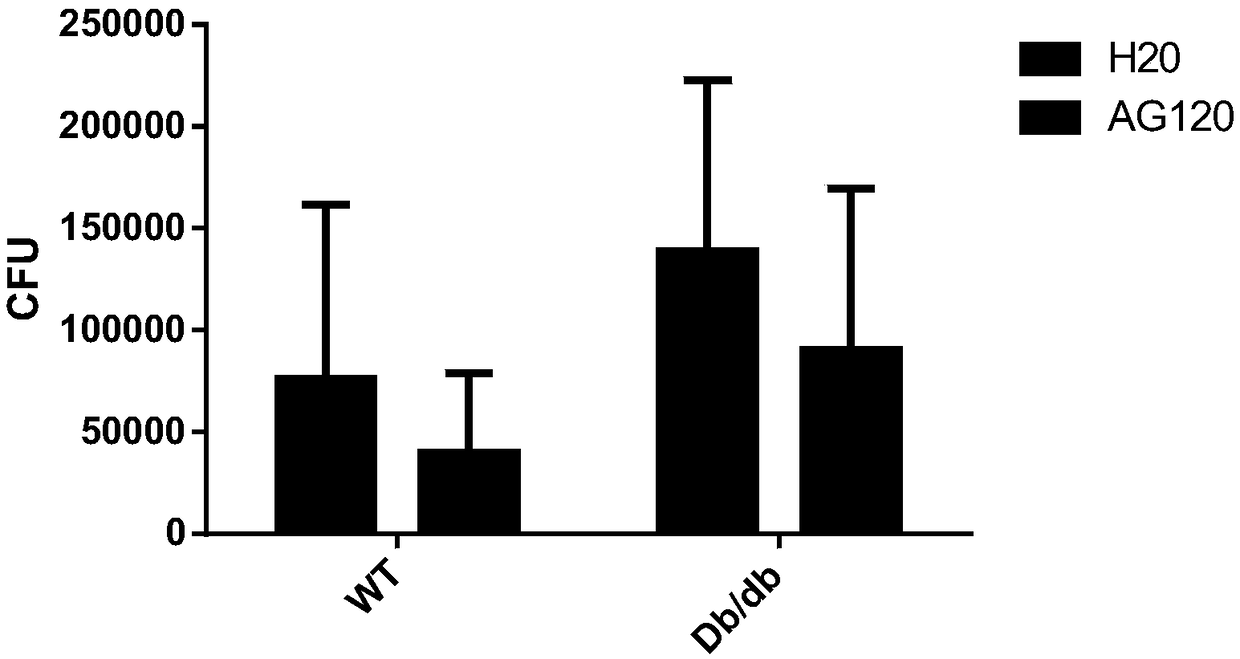
[0036] Take one-third of the mouse lung tissue after one week of infection and three weeks of administration in the above-mentioned Example 2, and grind it with 1 ml of PBS containing 1% triton-100, serially dilute, and take 10 -3 、10 -4 Spread 100ml of the tissue suspension evenly on the MiddleBook 7H10 agar culture plate containing amphotericin B, and then place it in a 37°C incubator for 2 to 3 weeks to complete the colony count. The specific results are as follows: image 3 shown.
[0037] The experimental results of this example confirmed that the compound AG120 significantly reduced the bacterial load of Mycobacterium tuberculosis in the lungs of mice.
[0038] Comprehensive analysis of the above examples shows that the compound AG120 can effectively inhibit the survival rate of Mycobacterium tuberculosis in vivo, thereby providing effective drug support for the treatment of tuberculosis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com