A class of ergostane steroid compound, its preparation method and use
A technology of steroidal compounds and ergosteranes, which is applied in the field of anti-AIDS drugs and can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
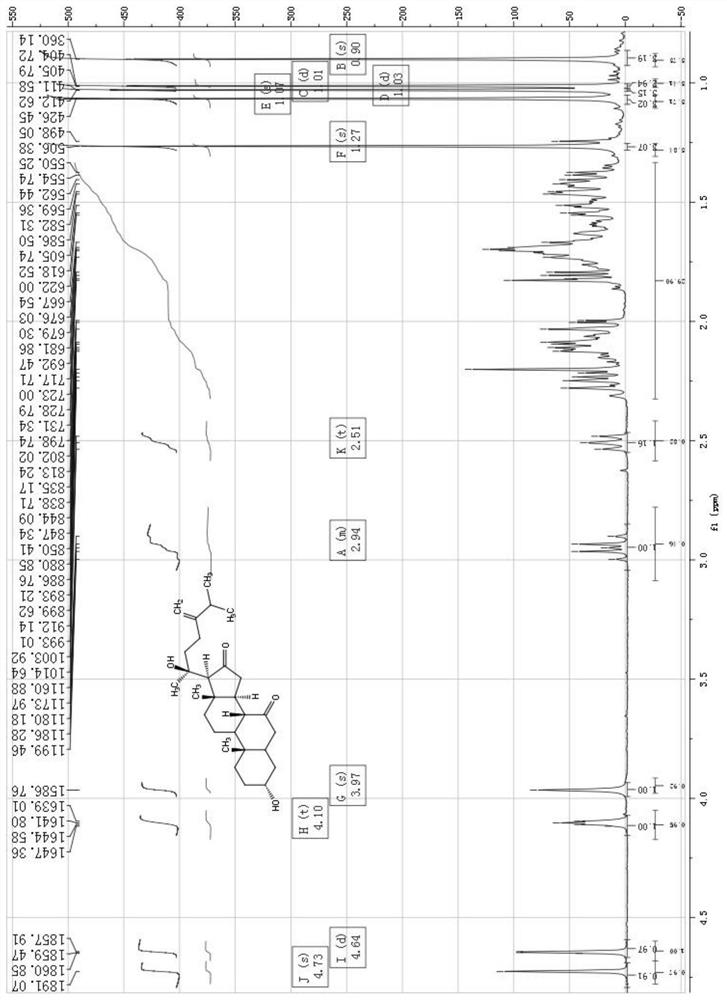
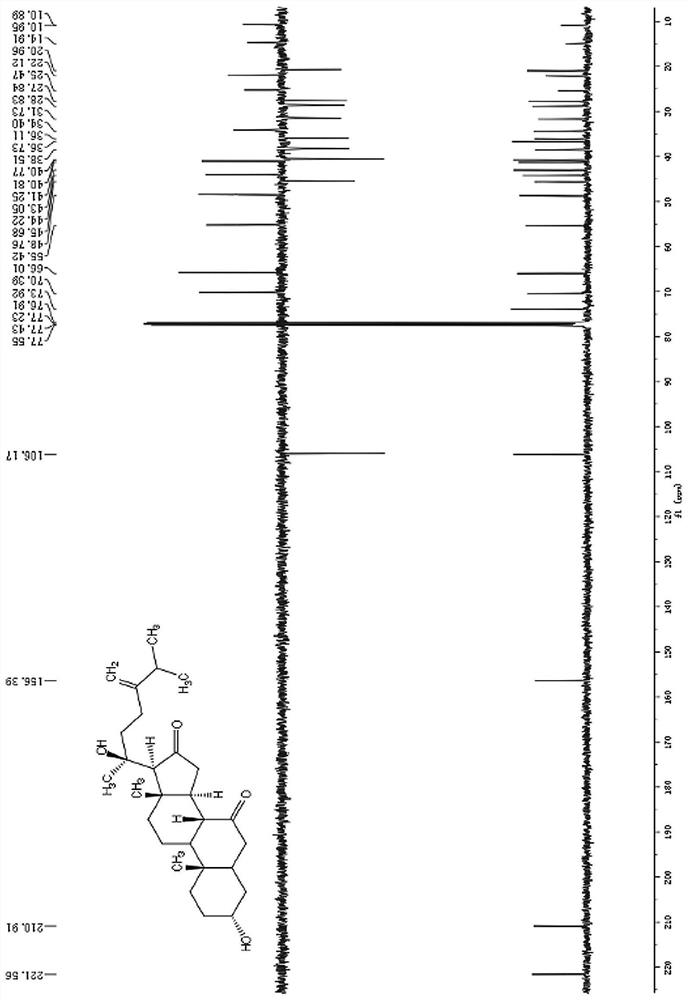
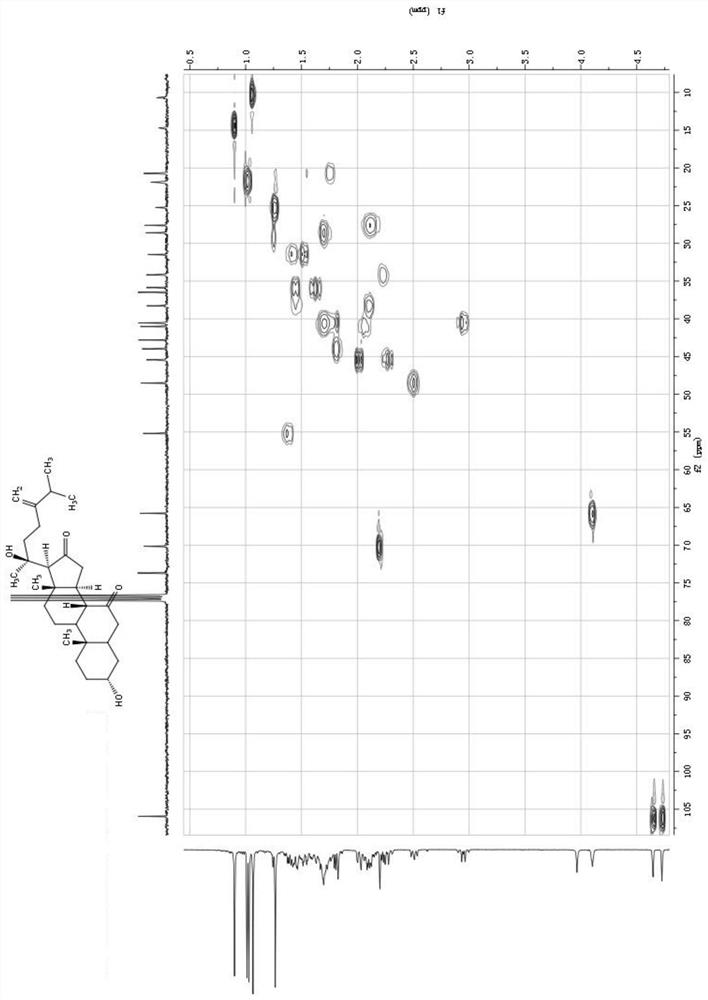
Embodiment 1
[0078] The preparation of compound 1-4 in the present invention in embodiment 1
[0079] Dry the whole herb powder (5Kg) with 95% ethanol at room temperature to extract 3 times, concentrate the extract under reduced pressure to obtain 300g extract, dilute with an appropriate amount of distilled water and extract 4 times with ethyl acetate, and concentrate the ethyl acetate part under reduced pressure Obtain 120g crude extract afterward, this crude extract is through MCI column gradient elution, and eluent is that the volume ratio is successively 4:6,5:5,6:4,7:3,8:2,9:1 The mixed solution of methanol and water, collect the eluate eluted by the mixed solution of methanol and water with a volume ratio of 6:4 and 7:3 to obtain component A (40g), collect the mixed solution of methanol and water with a volume ratio of 8:2 The solution eluted eluate afforded Fraction B (30 g).
[0080] Component A (40g) is subjected to silica gel column chromatography (200-300 mesh) and the volume r...
Embodiment 2
[0088] Anti-AIDS activity test of embodiment 2 compounds of the present invention
[0089] The anti-AIDS activity of compounds 1, 2, 3 and 4 prepared in Example 1 was tested.
[0090] 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide was purchased from Thermo Fisher Scientific; nevirapine, human acute lymphoblastic leukemia cells MT-4 cells , HIV NL4-3 virus was purchased from the National Institutes of Health AIDS Research and Reference Reagent Program https: / / www.aidsreagent.org; phosphate buffered saline, 10% v / v polyethylene glycol octylphenyl ether acid Isopropanol solution and formazan were purchased from Bioshop, Canada.
[0091] Anti-AIDS experiment: The anti-AIDS activity and the toxicity test in MT-4 cells induced by the compound were carried out with reference to the prior art method reported in Antiviral Chem.Chemother.2015, 24, 28-38, with nevirapine (NVP, nevirapine) as a positive control. MT-4 cells were added to 96-well plates containing compounds o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com