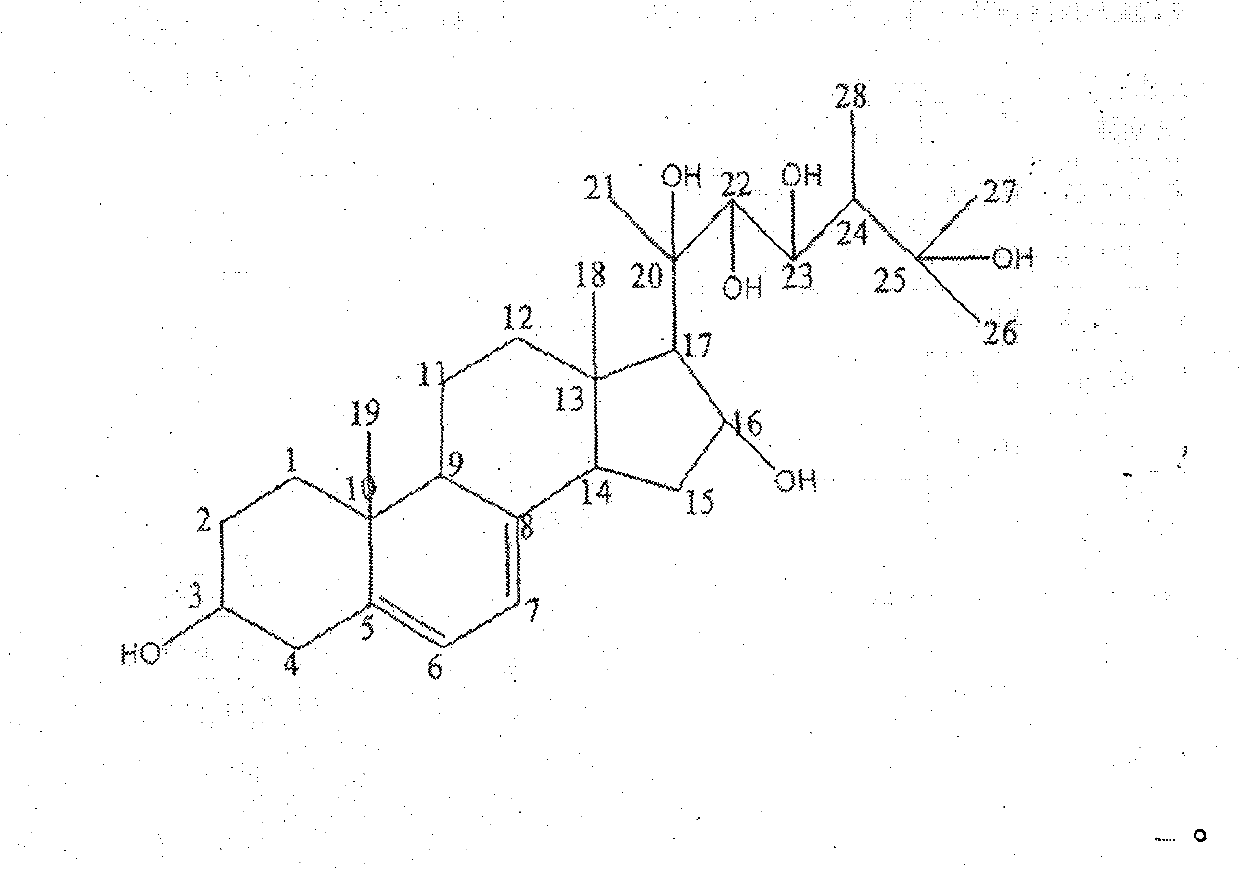
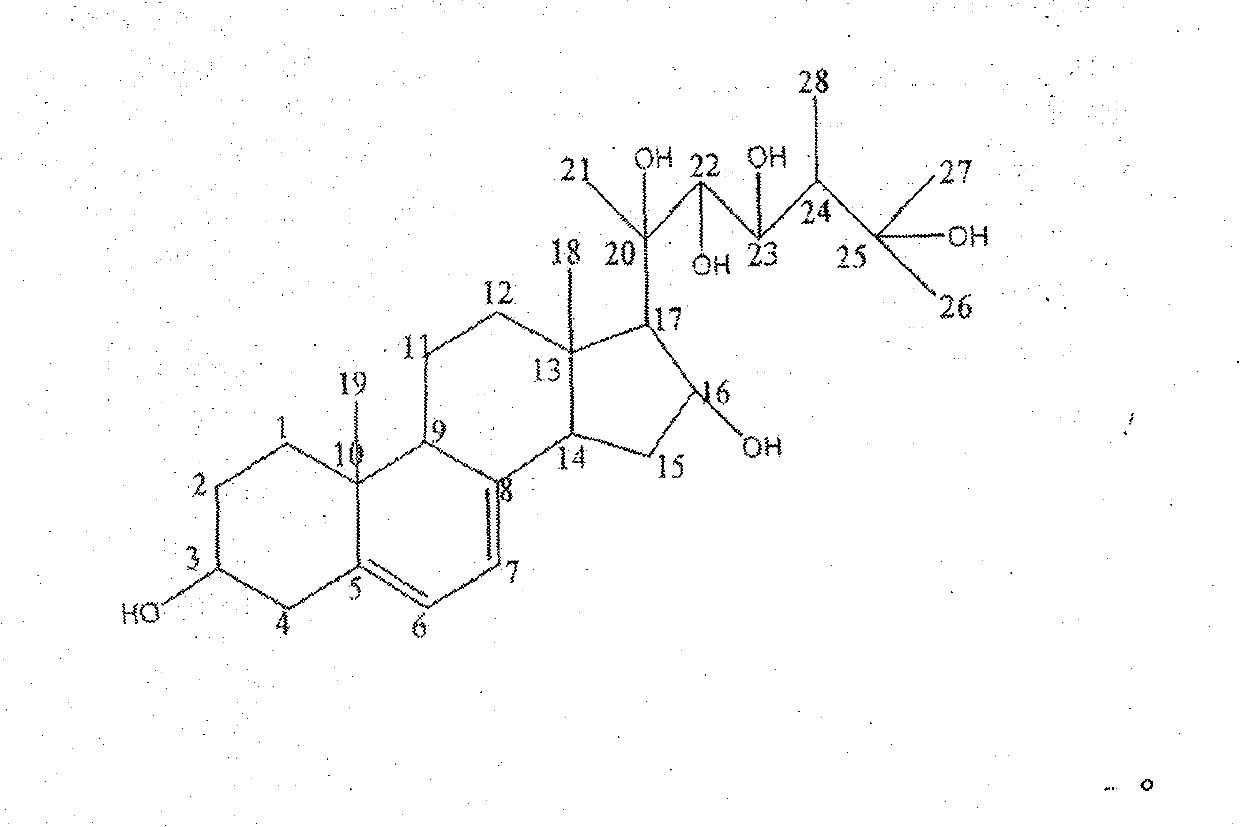
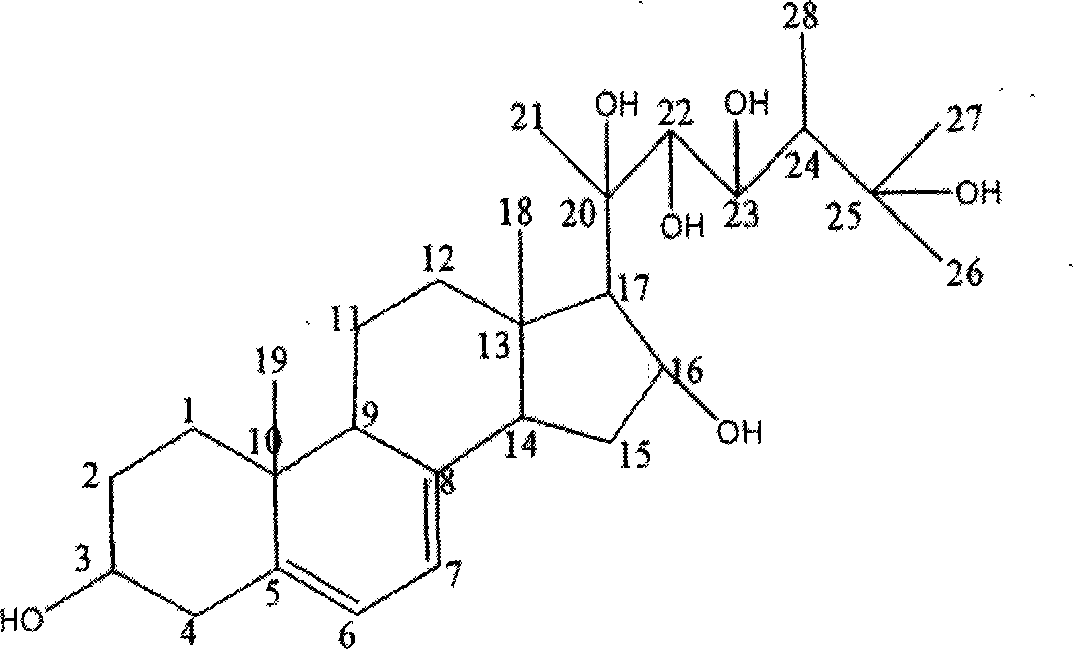
Novel compound and separation method thereof
A separation method and compound technology, applied in the direction of steroids, organic chemistry, drug combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0019] Experimental example 1 HMG-CoA reductase inhibitory activity test
[0020] The new compound 3, 16, 20, 22, 23, 25-hexahydroxyergoster obtained in Example 1 was dissolved in 75% ethanol, and the concentration was 2 mg / ml; the total volume in the assay system was 200 μl, each component Concentrations are: potassium chloride 200mM, potassium dihydrogen phosphate 160mM, ethylenediaminetetraacetic acid 4mM, dithiothreitol 10mM, two substrates nicotinamide adenine dinucleotide and 3-hydroxy-3-methylpentane The concentration of diacyl-CoA is 200 μM and 50 μM, pH6.8, add 20 μL of enzyme, add 5 μl of enzyme inhibitor, add 5 μl of 75% ethanol in the control group, and detect OD on a Versamax microplate reader at 37 °C 340 dynamic changes. By detecting OD 340 The speed of decline (indicated by slope value) within 5 minutes was used to evaluate the strength of HMG-CoA reductase activity, and then to evaluate the strength of inhibitory activity of enzyme inhibitors. The results ar...
Embodiment 1
[0026] Take 2000g of the contents of Xuezhikang capsules, and use petroleum ether, chloroform, and methanol, which are 4 times the weight of the contents of Xuezhikang capsules, as solvents for ultrasonic extraction. Each solvent is ultrasonically extracted 3 times for 30 minutes each time, and the extracts are combined to recover the solvent. , take petroleum ether part P, chloroform part C, methanol part M;
[0027] 250 g of part C was subjected to silica gel column chromatography and eluted with chloroform-methanol gradient to obtain pure chloroform eluted fraction C-I, 95:5 chloroform-methanol eluted fraction C-II, and 90:10 chloroform-methanol eluted fraction C- III, pure methanol elution part C-IV;
[0028] Part C-III was eluted with chloroform-methanol gradients of 90:10, 80:20, 70:30, and 50:50 respectively, and the part eluted with 80:20 chloroform-methanol was subjected to repeated silica gel column chromatography to obtain new compounds Crude product 0.047g; using ...
Embodiment 2
[0030] Take 1500g of the contents of Xuezhikang capsules, and use petroleum ether, chloroform, and methanol, which are 6 times the weight of the contents of Xuezhikang capsules, as solvents for ultrasonic extraction. Each solvent is ultrasonically extracted 3 times, each time for 20 minutes, and the extracts are combined to recover the solvent. , take petroleum ether part P, chloroform part C, methanol part M;
[0031] 300 g of part C was subjected to silica gel column chromatography and eluted with chloroform-methanol gradient to obtain pure chloroform eluted fraction C-I, 95:5 chloroform-methanol eluted fraction C-II, and 90:10 chloroform-methanol eluted fraction C- III, pure methanol elution part C-IV;
[0032] Part C-III was eluted with chloroform-methanol gradients of 90:10, 80:20, 70:30, and 50:50 respectively, and the part eluted with 80:20 chloroform-methanol was subjected to repeated silica gel column chromatography to obtain new compounds Crude product 0.042g; using...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com