A l-aspartate-α-decarboxylase with improved thermostability
A technology of aspartic acid and heat stability, applied in the field of genetic engineering, can solve the problem of low enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Construction of recombinant mutant Escherichia coli BL21 / pET28a-TcpanD
[0049]Using the L-aspartic acid-α-decarboxylase gene derived from the wild-type Tribulus chinensis as shown in SEQ ID NO.5 as a template, primers were designed:
[0050] For F49, the sequence information is shown in SEQ ID NO 9; for R49, the sequence information is shown in SEQ ID NO 10.
[0051] For F369, the sequence information is shown in SEQ ID NO 11; for R369, the sequence information is shown in SEQ ID NO 12.
[0052] For F221, the sequence information is shown in SEQ ID NO 13; for R221, the sequence information is shown in SEQ ID NO 14.
[0053] Use the whole plasmid PCR method to obtain the plasmid in which the mutated gene is connected to the expression vector pET 28a(+), digest it with DpnI for about 3 hours, and transfer it into Escherichia coli JM109 by competent heat stimulation. The plasmids were extracted overnight and sent to the sequencing company for sequencing. The p...
Embodiment 2
[0054] Example 2 Expression and Purification of Recombinant Mutant L-Aspartic Acid-α-Decarboxylase
[0055] Recombinant wild-type and mutant Escherichia coli BL21 / pET28a-TcpanD were inoculated in 5 mL of LB medium with a kanamycin concentration of 100 μg / mL, cultured overnight at 37°C with shaking at 200 r / min. Inoculate the above overnight culture into 2YT medium containing 100 μg / mL of kanamycin at an inoculum size of 1%, culture at 37°C with shaking at 200 r / min until the OD600 of the bacterial solution reaches 0.6-0.8, and add IPTG to the final concentration 0.2mmol / L, induced culture at 20°C for 16-20h, collected the bacteria and sonicated, and analyzed and identified the expression level of L-aspartic acid α-decarboxylase recombinant protein by Tris-tricine SDS-PAGE method. Proteins were purified by sonication, centrifugation, and His Trap FF affinity chromatography column.
Embodiment 3
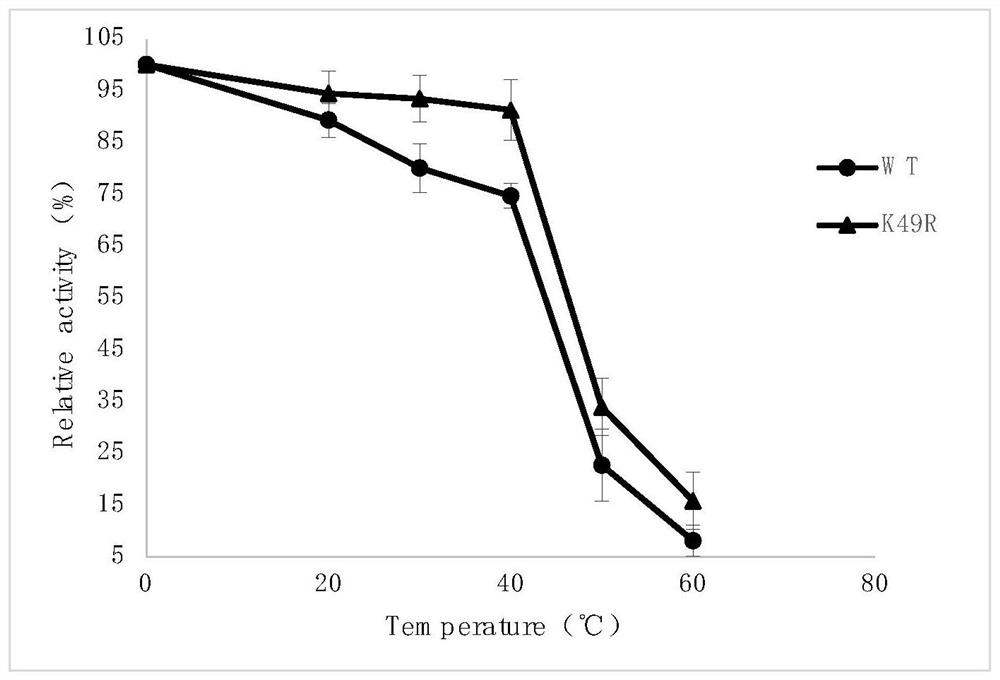
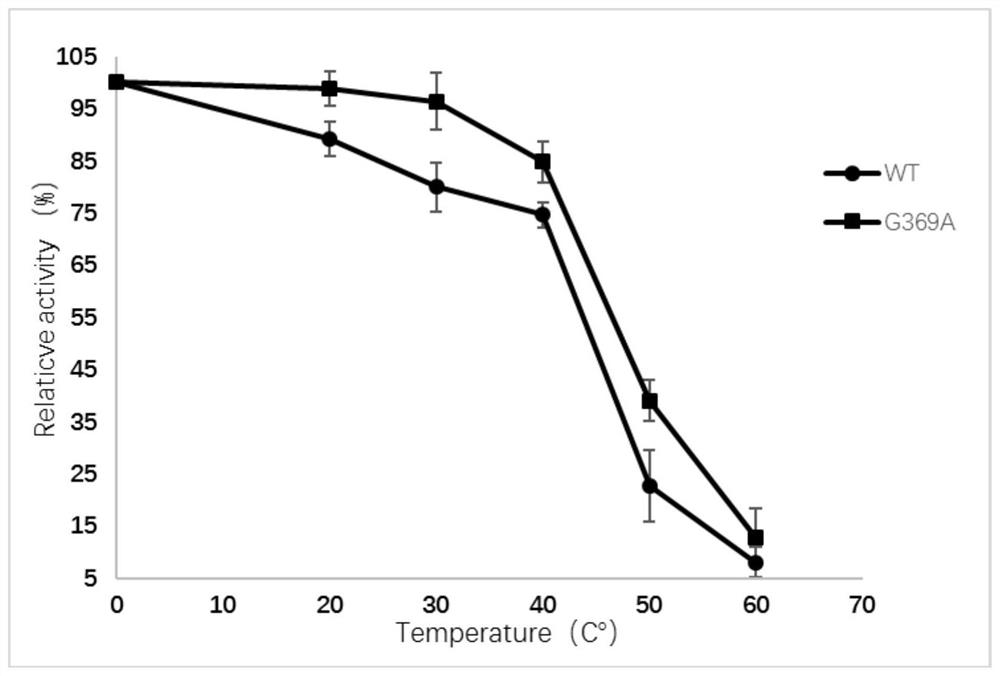
[0056] Example 3 Recombinant Mutant Enzyme Activity and Thermal Stability Detection
[0057] The L-aspartic acid α-decarboxylase recombinant Escherichia coli was collected and purified with an affinity chromatography column. After the target protein was detected by SDS-PAGE gel electrophoresis, the concentration of the target protein was measured by the Brandford method.
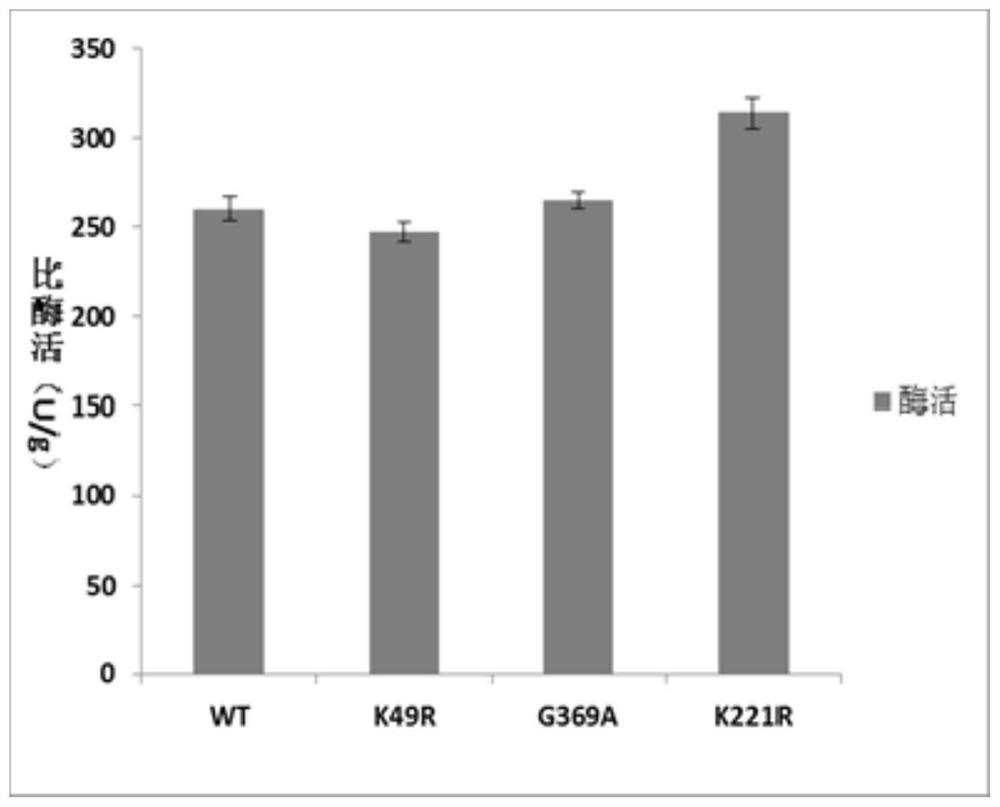
[0058] (1) Comparison of enzyme activity:
[0059] Definition of enzyme activity: at 37°C and pH 6.5, the amount of enzyme required to convert 1 mM product β-alanine per hour is defined as 1U.
[0060] Definition of specific enzyme activity: the number of enzyme activity units per gram of protein.
[0061] Determination of L-aspartic acid-α-decarboxylase activity: A large number of Escherichia coli capable of normal expression were cultured, mature cells were collected by centrifugation, resuspended with 50mM phosphate buffer (pH 6.5), and resuspended twice by repeated centrifugation. After several times,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com