Aspergillus nidulans chitin deacetylase, and preparation method and application thereof
A technology of deacetylase and Aspergillus nidulans, applied in the field of Aspergillus nidulans chitin deacetylase and its preparation, can solve the problems of limited development, lack of chitin deacetylase preparations, etc., and achieve the effect of high biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Codon optimization and total gene synthesis of chitin deacetylase gene
[0032]On the premise of not changing the amino acid sequence, the chitin deacetylase of Aspergillus nidulans was artificially designed using the preferred codon of Pichia pastoris (as shown in the sequence SEQ ID NO.1, GenBank accession number: XP_682649.1) The coding gene, the specific nucleotide sequence is shown in SEQ ID NO.2. The homology between the optimized nucleotide sequence and the original coding gene sequence (as shown in SEQ ID NO.3, GenBank accession number: XM_677557.1) is 72%. The optimized gene sequence was entrusted to Beijing Qingke Xinye Biotechnology Co., Ltd. for the total synthesis, and the synthesized gene sequence was named chitin deacetylase gene ancdal.
Embodiment 2
[0033] Example 2 Construction of expression vector of chitin deacetylase gene ANCDA1
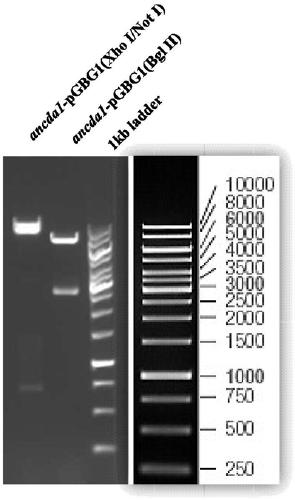
[0034] The signal peptide sequence shown in SEQ ID NO.4 in the expression vector pPIC9 was replaced by the signal peptide sequence shown in SEQ ID NO.5 by using Nsi I / Xho I double enzyme digestion to obtain the expression vector pGBG1. Use restriction endonucleases Xho I and Not I to double-enzyme digest the cloning vector containing chitin deacetylase gene ancda1 to obtain the target gene fragment, and use the same endonuclease to double-enzyme digest the expression vector pGBG1, Recycle large fragments. The two recovered products were connected to obtain a recombinant vector named ancda1-pGBG1. In order to confirm that the target chitin deacetylase gene has been constructed into the vector, we use Xho I / Not I and Bgl II to perform double digestion and single digestion of the recombinant vector, and perform agarose gel electrophoresis on the product. The result is as figure 1 Shown: afte...
Embodiment 3
[0035] Example 3 Screening of chitin deacetylase Pichia pastoris engineered bacteria and preparation of chitin deacetylase
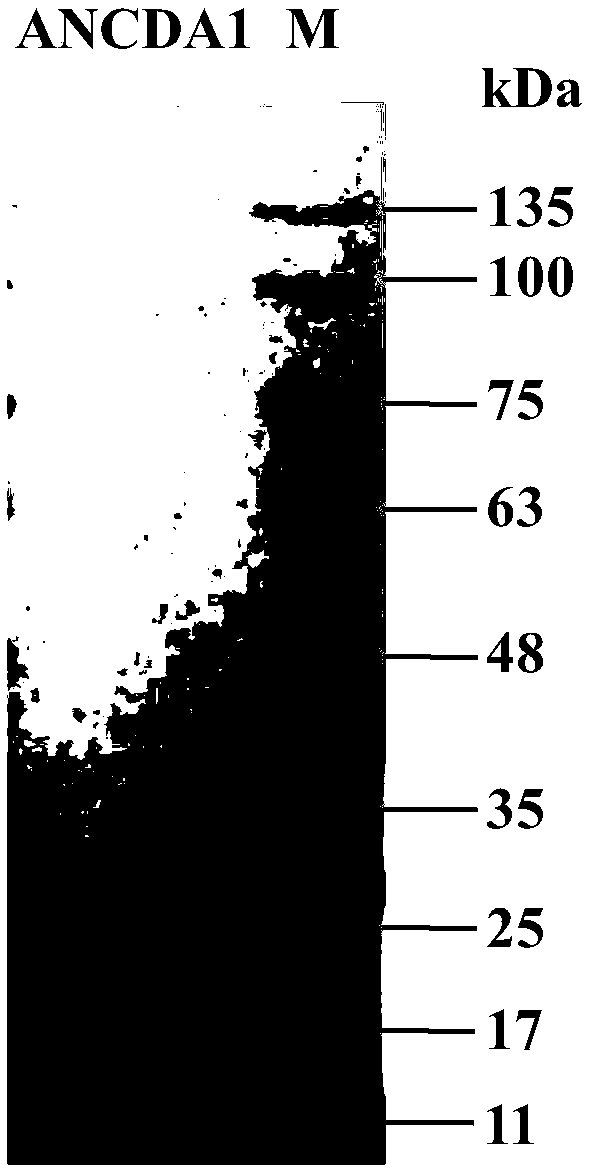
[0036] After linearizing the obtained recombinant plasmid ancda1-pGBG1 with restriction endonuclease Bgl II, gel electrophoresis was used to separate and excise the nucleotide fragment containing the target gene (such as figure 1 The larger fragment shown) was introduced into Pichia pastoris GS115 by electroporation, and the obtained recombinants were screened on the histidine auxotrophic MD plate. Pick 6 single colonies and inoculate them in 200mL BMGY medium, culture at 30°C and 250rpm for 48 hours, centrifuge to discard the supernatant, and add an equal amount of BMMY to induce expression. After 24 hours, methanol was added to a final concentration of 1%, and added every 24 hours thereafter. After a total of 120 hours of induction, centrifugation was performed, and SDS-PAGE was used to detect the expression of the target protein in the protein superna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com