Monoclonal antibody capable of recognizing HPV18 and/or HPV45, and applications thereof
An antibody and carrier technology, applied in applications, antibodies, antiviral agents, etc., can solve the problems of uncertain treatment plan, inconsistent interpretation of CIN2, and difficulty in accurate diagnosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0166] Preparation of monoclonal antibodies
[0167] Antibodies of the present invention can be prepared by various techniques known to those skilled in the art. For example, an antigen of the invention may be administered to an animal to induce the production of monoclonal antibodies. For monoclonal antibodies, hybridoma technology can be used to prepare (see Kohler et al., Nature 256; 495, 1975; Kohler et al., Eur.J.Immunol.6:511, 1976; Kohler et al., Eur.J.Immunol. 6:292,1976; Hammerling et al., In Monoclonal Antibodies and T Cell Hybridomas, Elsevier, N.Y., 1981), phage display technology or available recombinant DNA method (US Patent No. 4,816,567).
[0168] Representative myeloma cells are those that fuse efficiently, support stable high-level production of antibody by selected antibody-producing cells, and are sensitive to culture medium (HAT medium matrix), including myeloma cell lines, such as murine Myeloma cell lines, including those derived from MOPC-21 and MPC-1...
Embodiment 1
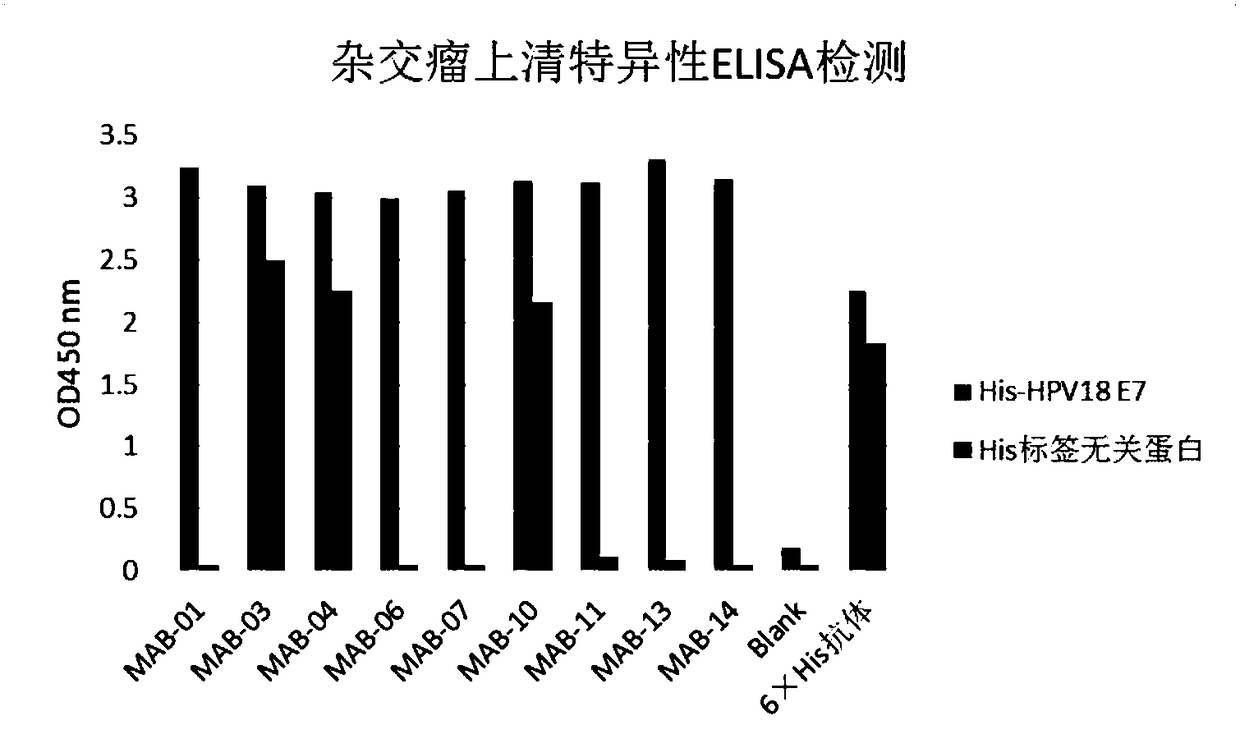
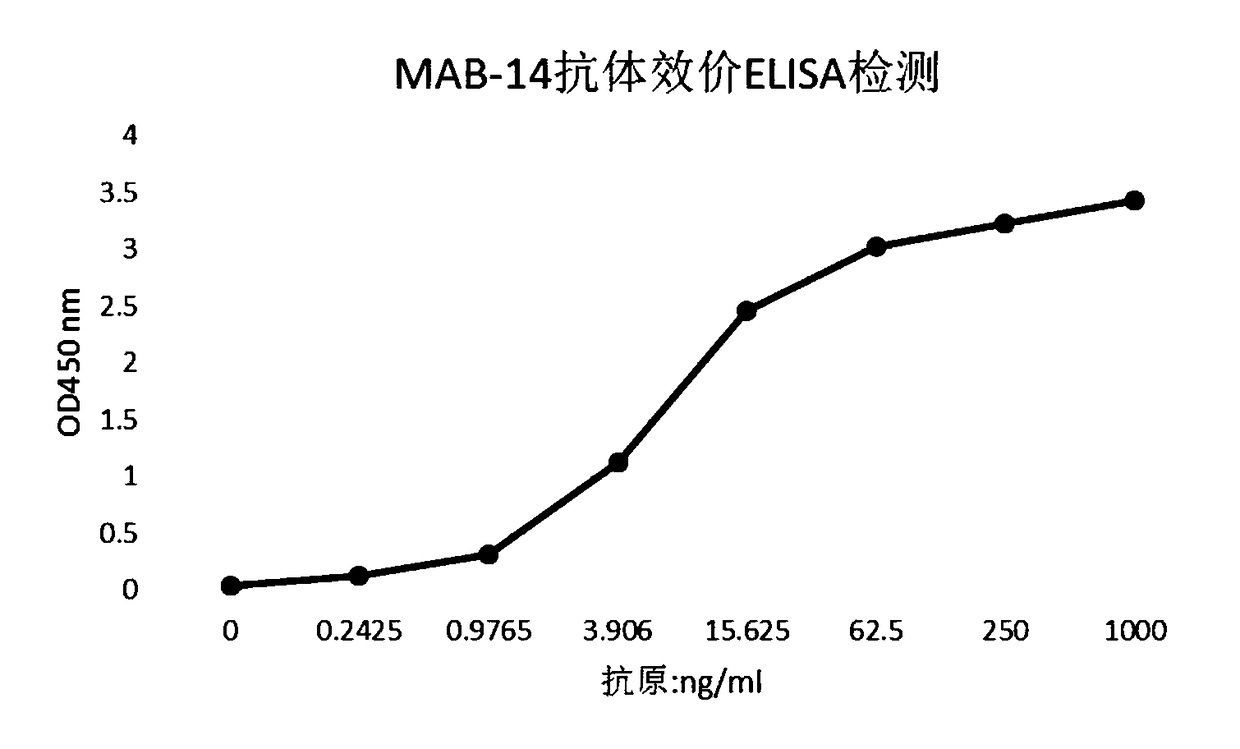
[0203] 1 Preparation of human papillomavirus HPV18 E7 monoclonal antibody
[0204] 1.1 Animal immunity
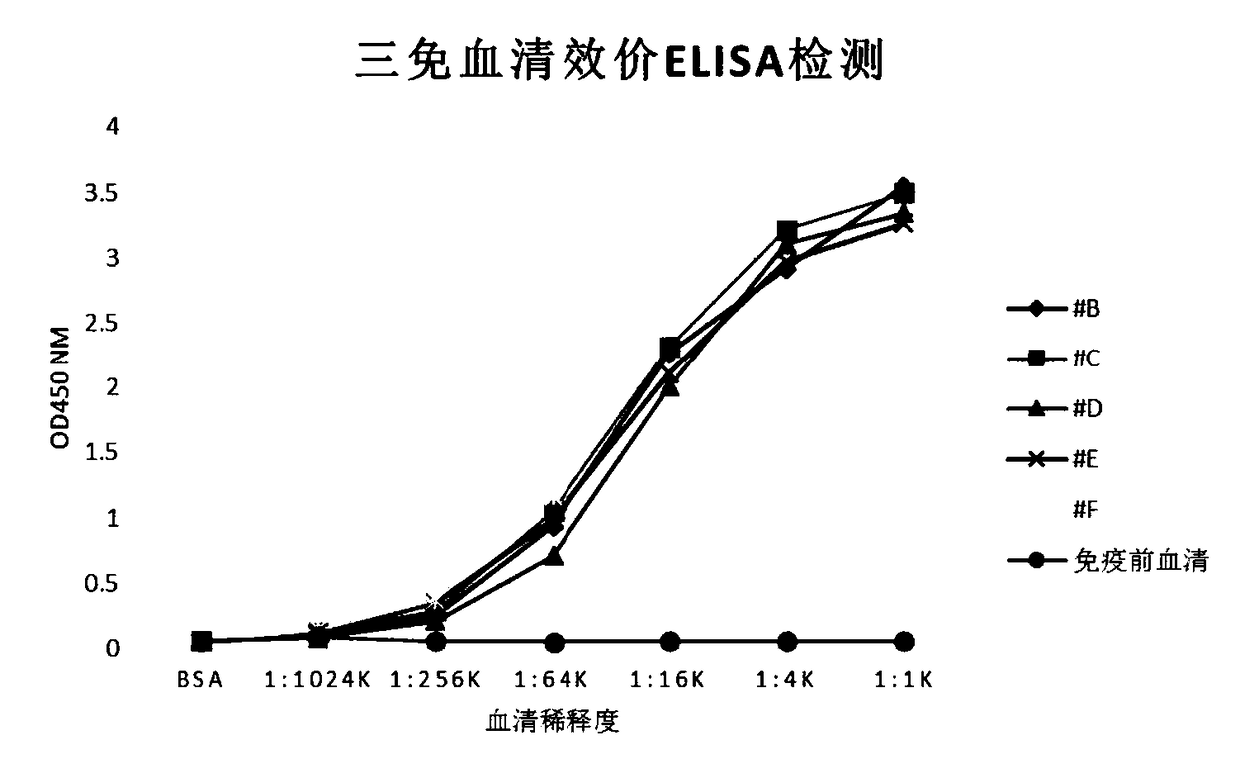
[0205] Five 4-6-week-old female BALB / C mice were taken, and the immunogen was His-HPV18 E7 protein. The immunization procedures are shown in Table 1. Before each immunization, the blood was collected by docking the tail of the mice, and the His-HPV18 E7 recombinant protein (5 μg / ml) was used as the detection antigen to coat the indirect ELISA method to detect the serum titer of the mice. The titer titer of the serum of the immunized mice was >1: At 10,000 splenocytes were taken for fusion. Results #F mouse serum had the highest titer and reached the fusion standard ( figure 1 ), and #F mice were taken for fusion.
[0206] Table 1 Mouse immunization program
[0207]
[0208] 1.2 Cell Fusion and Culture
[0209] The splenocytes of immunized mice were collected and fused with myeloma cells SP2 / 0 according to conventional methods. The fusion ratio of splenocytes: SP2 / 0=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com