Application of platelet-related inhibitors in the preparation of drugs for treating thrombocytopenia
A technology for thrombocytopenia and inhibitors, applied in the field of application in the preparation of drugs for treating thrombocytopenia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
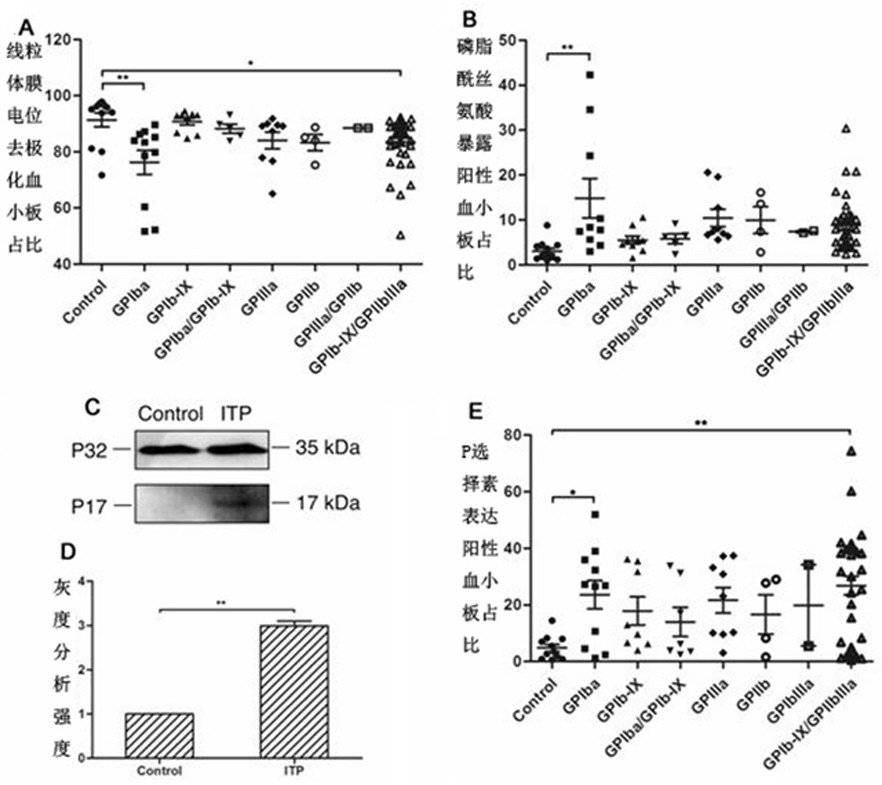
[0027] The plasma of ITP patients is classified according to the different antibodies it contains and then incubated with normal human platelets for detection. figure 1 for the result. The results showed that, compared with the control, the plasma of anti-GPIbα antibody-positive ITP patients could reduce the mitochondrial membrane potential of normal human platelets ( figure 1 A), increased expression of P-selectin on the platelet membrane surface ( figure 1 E), with increased platelet membrane phosphatidylserine eversion ( figure 1B), and in the corresponding activation and apoptosis indicators, ITP patient plasma containing anti-GPIIbIIIa antibody did not lead to a decrease in platelet mitochondrial membrane potential ( figure 1 A), increased P-selectin expression ( figure 1 E), PS eversion ( figure 1 b); figure 1 C. figure 1 D can also see the difference.
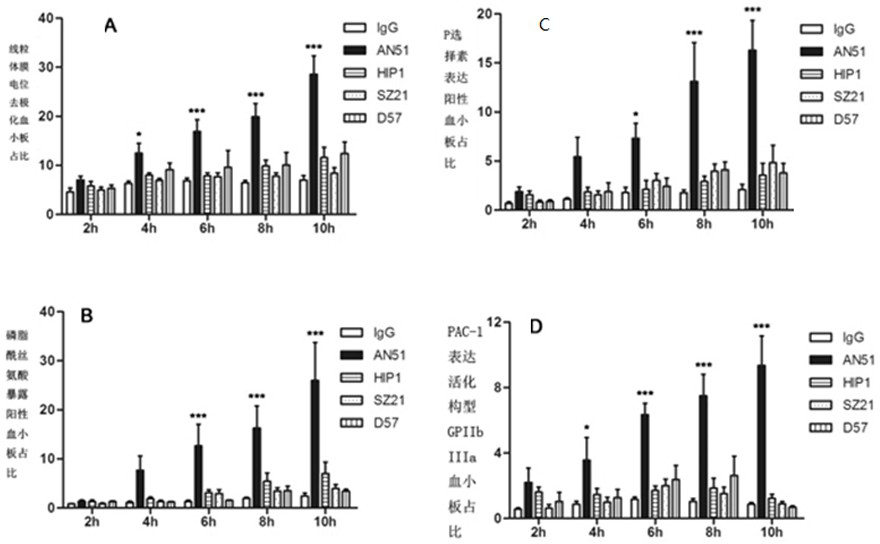
[0028] Human washed platelets were incubated with 10ug / ml antibody IgG, anti-GPIbα antibody AN51, anti-GPIbα an...
Embodiment 2
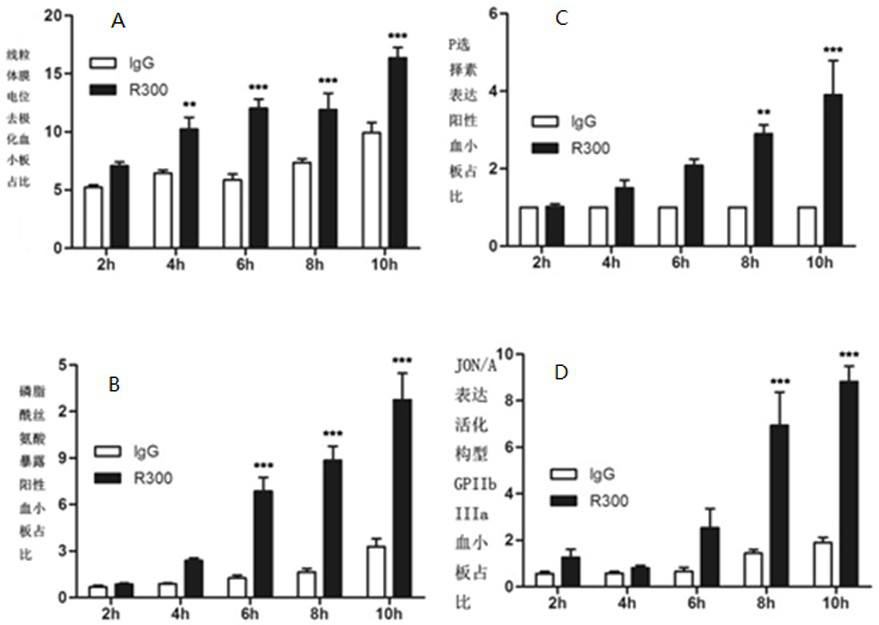
[0031] Human washed platelets were pre-incubated with GPIbα cluster inhibitor GlcNAc (100mM) and GM3 (100uM) for 15 minutes at room temperature, activation inhibitor BAPTA (10uM) at 37°C for 15 minutes, apoptosis inhibitor Q-VD-Oph (100uM)37 After 30 minutes at ℃, AN51 10ug / ml was added to all groups, and PS valgus was detected after incubating at 37 ℃ for 8 hours. Similarly, mouse PRP was incubated with GPIbα cluster inhibitor GlcNAc (100mM) and GM3 (100uM) in advance at room temperature. After 15 minutes, activation inhibitor BAPTA (20uM) at 37°C for 15 minutes, apoptosis inhibitor Q-VD-Oph (100uM) at 37°C for 30 minutes, add R300 5ug / ml, and incubate at 37°C for 6 hours to detect PS eversion, mitochondrial membrane potential, P-selectin expression, see Figure 4 , the results showed that GlcNAc, GM3, BAPTA, Q-VD-Oph can inhibit the AN51-induced platelet activation and apoptosis common pathway PS eversion ( Figure 4 A); the mouse results are the same as the human results, ...
Embodiment 3
[0033] Fluorescent secondary antibody and control antibody, R300, R300F(ab) 2 After mixing, the mice were injected intraperitoneally, and the tissues and organs were taken out after 4 hours for observation by in vivo imaging of small animals; control antibody, R300, F(ab) were injected intraperitoneally, respectively. 2 After 4 hours, the liver was taken for frozen section, and the nuclei were stained with F4 / 80 (green) labeled macrophages, GPIbα (red) labeled platelets, and DAPI (blue), and the results were as follows Figure 5 shown.
[0034] Figure 5 A can see R300 and R300 F(ab) 2 The bound platelets are mainly accumulated in the liver; Figure 5 The results of B showed that platelets mainly co-localized with macrophages; Figure 5 C shows that the removal of macrophages with clodronate disodium liposome can rescue the thrombocytopenia caused by intraperitoneal injection of R300; Figure 5 D shows that the removal of macrophages can significantly reduce the removal o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com