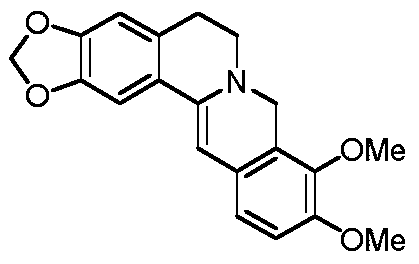
A kind of preparation method of dihydroberberine
A technology of dihydroberberine and donor, which is applied in the field of preparation of dihydroberberine, can solve the problems of only 55% separation yield, increase of hydrolyzed impurities, excessive yield, etc., and achieve convenient and easy post-reaction treatment Good control and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0029] The preparation of embodiment 1 dihydroberberine
[0030]
[0031] Under nitrogen protection, berberine hydrochloride (10g, 26.9mmol) was dissolved in 150mL of methanol, and 0.1g of 10% palladium carbon, ammonium formate (5.08g, 80.7mmol) and sodium bicarbonate (2.25g, 26.9mmol) were added at room temperature ). After the addition is complete, stir at 40-50°C for 5 hours, and TLC detects that the reaction is complete. After filtration, it was concentrated, slurried with water and then dried to obtain 8.9 g of yellow solid (molar yield: 98%). The liquid phase purity is 98%. 1 H NMR (300MHz, CDCl 3 )δ7.18(d,1H,J=8.7Hz),6.73(m,2H),6.56(s,1H),5.95(s,1H),5.94(s,2H),4.32(s,2H), 3.84(s, 6H), 3.20(t, 2H, J=8.1Hz), 2.90(t, 2H, J=8.1Hz).
Embodiment 2 2
[0032] The preparation of embodiment 2 dihydroberberine
[0033]
[0034] Under nitrogen protection, berberine nitrate (10 g, 25.1 mmol) was dissolved in 150 mL of isopropanol, and 0.1 g of Raney nickel and potassium carbonate (2.08 g, 15.1 mmol) were added at room temperature. After the addition is complete, stir at 70-80°C for 12 hours, and TLC detects that the reaction is complete. Concentrate after filtration, beat with water and dry to obtain 8.0 g of yellow solid (molar yield: 95%). The liquid phase purity is 98%.
Embodiment 3 2
[0035] The preparation of embodiment 3 dihydroberberine
[0036]
[0037] Berberine acetate (10 g, 25.2 mmol) was dissolved in 150 mL of tetrahydrofuran, and 0.1 g of 10% platinum on carbon and sodium hydroxide (1.1 g, 26.5 mmol) were added at room temperature. After the addition was completed, the mixture was stirred at 20-30° C. under normal pressure for 8 hours under a hydrogen atmosphere, and the reaction was detected by TLC. Concentrate after filtration, beat with water and dry to obtain 8.0 g of yellow solid (molar yield: 95%). The liquid phase purity is 98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com