A photocatalyst cu 3 vs 4 preparation method
A photocatalyst, CH4N2S technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of expensive reaction raw materials, harsh environmental conditions, expensive raw materials and equipment requirements, etc., to achieve reaction Mild conditions, good catalytic effect, and economical reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
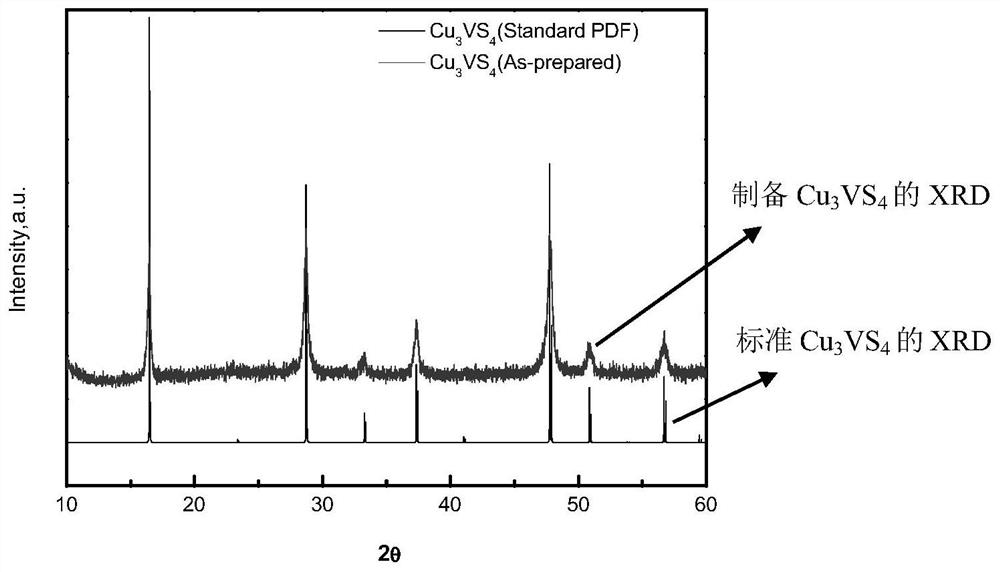
[0023] Embodiment 1 photocatalyst Cu 3 vs. 4 preparation of
[0024] 1) The amount of the substance weighed on a ten-thousandth balance is 3mmol copper chloride dihydrate (CuCl 2 2H 2 O), 1mmol ammonium metavanadate (NH 4 VO 3 ) and 20mmol thiourea (CH 4 N 2 S), the weighed raw materials were mixed and ground together in an agate mortar, and then added into a hydrothermal reaction kettle, and the reaction kettle was reacted at 230° C. for three days.
[0025] 2) Cool naturally after the reaction is over, wash the product in the kettle with distilled water: first wash the product in the inner lining with a small amount of distilled water, then take it out and place it in a beaker, wash it 3 times under stirring, stir and let it stand, take the solid in the lower layer and put it in the beaker at 60 Dry at ℃ to obtain the product.
[0026]3) After the product is dried, measure the quality of the product on a ten-thousandth balance and record it, that is, the actual outpu...
Embodiment 2
[0029] Embodiment 2 catalytic efficiency test
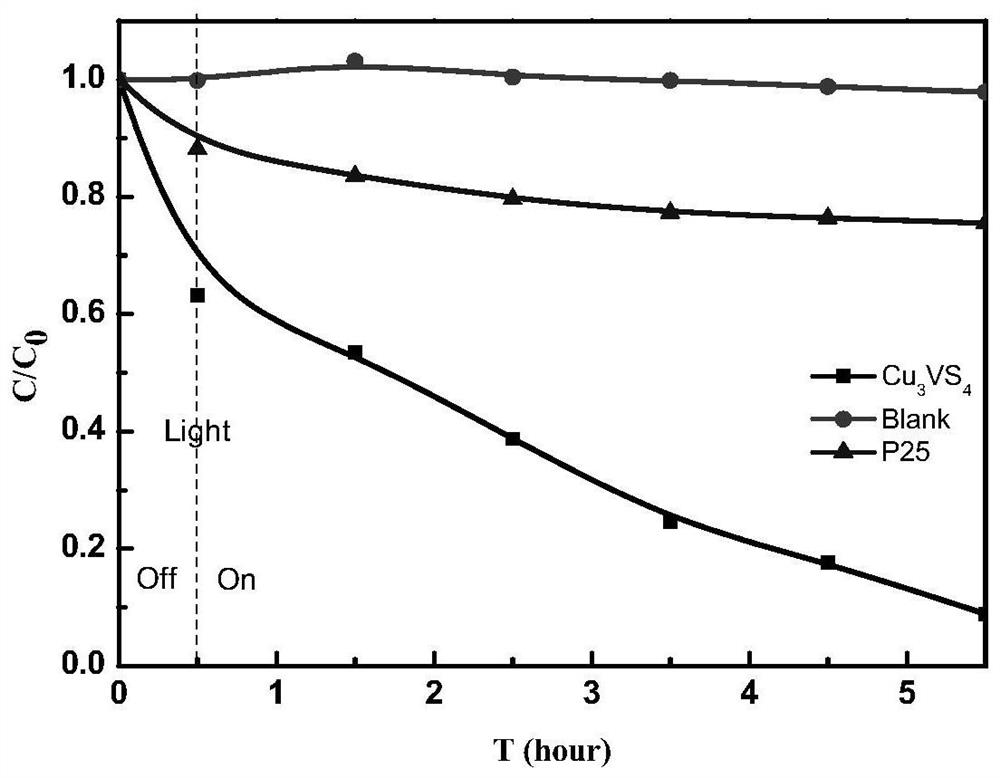
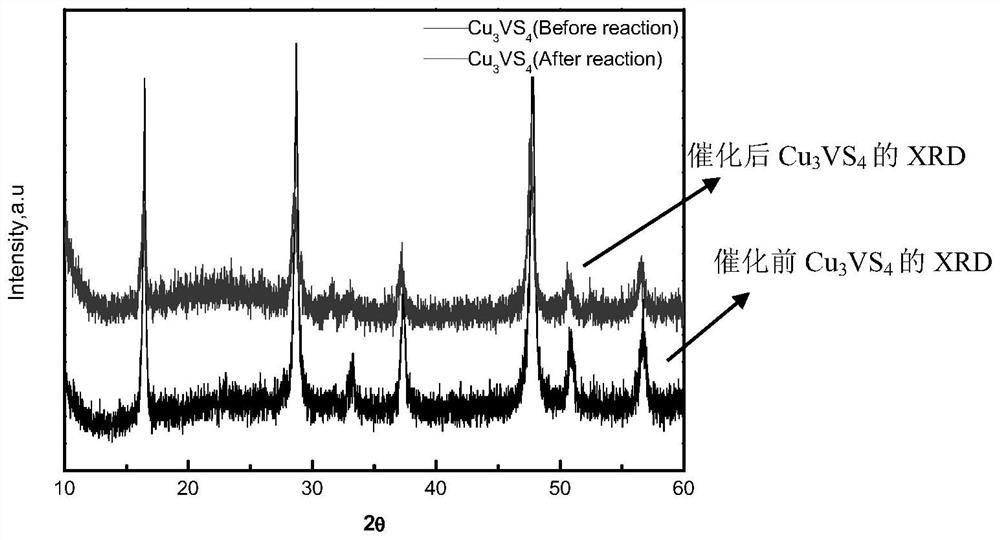
[0030] 1) Through the experiment of photocatalytic degradation MO dye, test the photocatalyst Cu prepared by the method of the present invention 3 vs. 4 It has a degradative effect on MO dyes, and the results show that the catalytic performance in the visible light region is better than that of titanium dioxide (P25), and it has good stability in dyes.
[0031] 2) The specific steps of the experiment are as follows:
[0032] The Cu that embodiment 1 makes 3 vs. 4 Take 25 mg and add it to 50 ml of MO dye with a concentration of 10 mg / L and PH=3 to form a mixed solution, and absorb it in the dark for 30 min to reach adsorption equilibrium, and then illuminate it for 5 h under a 125W ultraviolet high-pressure mercury lamp (filter figure 2 ) to calculate the degradation rate. by Cu 3 vs. 4 Catalyst P25 of the same quality and the blank group experimental result without catalyst are contrasted (such as figure 2 ), to analyze ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com