Compositions, medicaments and sgRNAs for increasing coagulation activity in hemophilia b patients
A technology of hemophilia and composition, applied in the direction of recombinant DNA technology, DNA/RNA fragments, drug combination, etc., can solve the problem of high price, reduce toxic side effects, reduce the dose of virus vector, improve completeness and stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
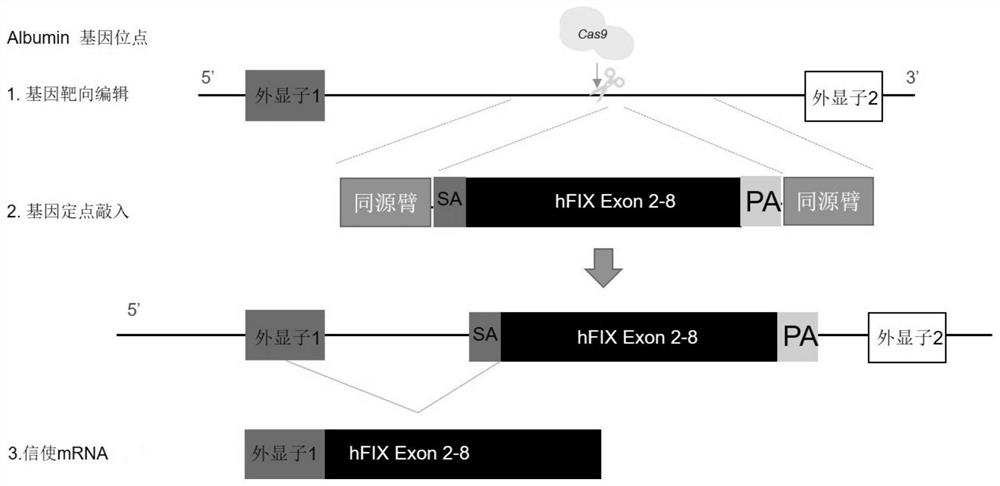
[0052] Construction of a vector targeting the intron 1 region of the Albumin gene and capable of directional integration of functional coagulation factor IX (hereinafter referred to as F9)
[0053] 1. Strategies for directional integration of functional coagulation factor IX in the intron 1 region of the Albumin gene using the CRISPR / Cas9 gene editing system figure 1 shown.
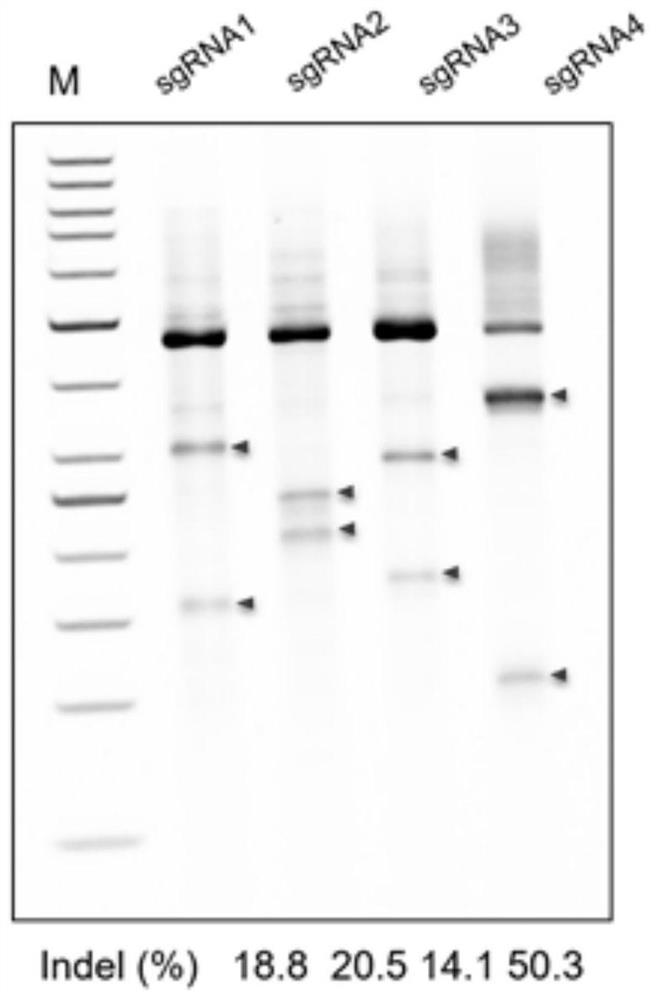
[0054] 2. sgRNA gene editing efficiency verification
[0055] 2.1 According to the sequence of the intron 1 region of the Albumin gene, sgRNAs targeting different sites in the intron 1 region of the Albumin gene were designed. The sequences of the target sites targeted by each sgRNA are shown in Table 1.
[0056] Table 1
[0057]
[0058] 2.2 According to the target site sequence in Table 1, synthesize each sgRNA oligonucleotide single strand (see Table 2), add 4 bases to the 5' end of the sense and antisense strands during synthesis, and make it compatible with the pX330 vector (vector schematic dia...
experiment example 1
[0080] Detect the expression level of F9 treated with the above-mentioned AAV8.CRISPR-SpCas9 vector system
[0081] Experimental group:
[0082]Targeted therapy group: inject the first AAV virus vector AAV8.SpCas9 (1 × 10 12 genome copy / mouse) and the second AAV viral vector AAV8.sgRNA.donor (3×10 12 Genome copies / mouse) of 8-week-old female hemophilia B mice (n=5);
[0083] Non-targeting control group: AAV8.SpCas9 (1×10 12 Genome copy / mouse) and AAV8.control.donor (empty vector, no expression of sgRNA, 3×10 12 Genome copies / mouse) of 8-week-old female hemophilia B mice (n=5);
[0084] Untreated group: 8-week-old female hemophilia B mice without any treatment (n=5);
[0085] Blood samples were taken at different time points after treatment to detect the expression level and function of F9.
[0086] Detection method: Take blood from the orbit and dilute it with 3.2% sodium citrate anticoagulant 10:1, then centrifuge to get the supernatant plasma, divide it into two parts,...
experiment example 2
[0089] The efficiency of targeted integration of the F9 gene after treatment in hemophilia B mice was examined.
[0090] The experimental grouping is the same as in Experimental Example 1.
[0091] After the treatment, the liver lobe was washed with pre-cooled PBS, absorbed to remove water, placed in OCT embedding medium, frozen in liquid nitrogen, sliced with a cryostat (section thickness 8 μm), fixed, and processed with goat anti-human F9 antibody. Immunofluorescence staining, using ImageJ software to take pictures and analyze the experimental results. Liver homogenate was collected, tissue RNA was extracted, cDNA was synthesized by reverse transcription, target-specific qPCR primers were designed, and Sybr Green qPCR kit was used for fluorescent quantitative PCR detection, and Albumin-specific qPCR was used as a control.
[0092] After the experiment, the liver tissue was harvested, the whole genomic DNA was extracted by homogenization, the genomic DNA was treated with X...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com