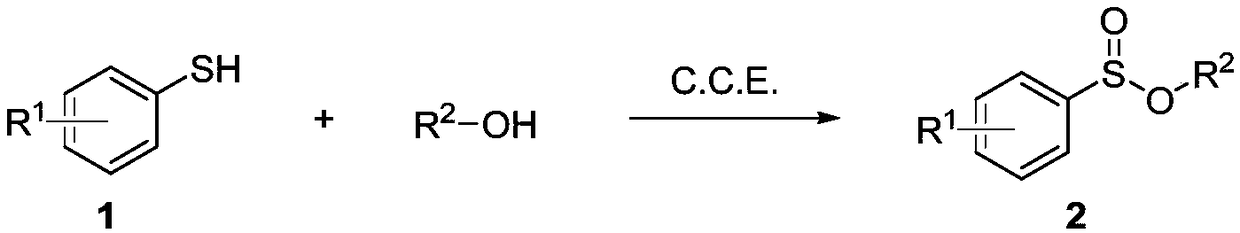
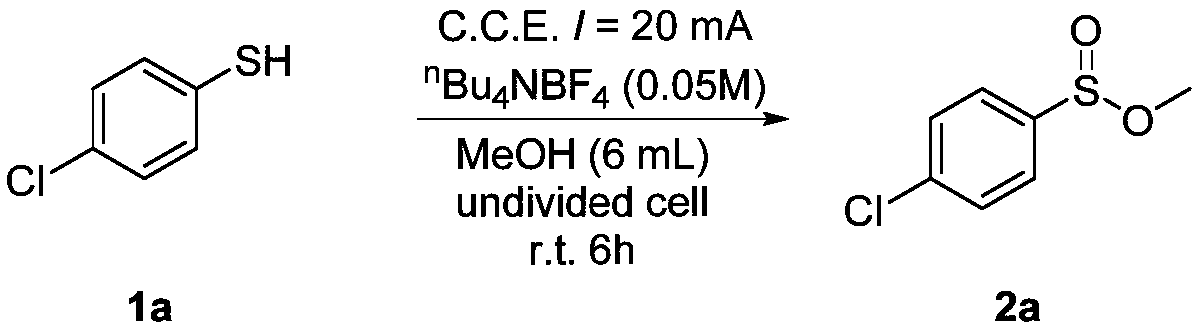Electrochemical synthesis method of aryl sulfuric acid ester compound
A technology of aryl sulfinate and synthesis method, which is applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., to achieve the effects of wide substrate applicability, simple and safe operation, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Add 4-chlorothiophenol (0.5mmol) and tetra-n-butyl ammonium tetrafluoroborate (0.3mmol) to a 25mL reaction tube equipped with a magnetic stirring bar, and then add 6.0mL methanol; fix the reaction tube on a magnetic stirring Add electrodes (graphite cathode, graphite anode) to conduct 20mA constant current electrolysis, and at the same time stir the reaction solution evenly; after the mixture reacts at room temperature (25°C) for 6h, the reaction ends; the solvent is removed with a rotary evaporator, and the crude product is passed through the column Chromatography (petroleum ether: ethyl acetate = 20:1) separation and purification gave the target product (2a) with a yield of 90%. The NMR data of this compound are: 1 H NMR (600MHz, CDCl 3 )δ7.63(d, J=8.6Hz, 2H), 7.50(d, J=8.5Hz, 2H), 3.46(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ142.42, 138.73, 129.45, 126.96, 49.87.
Embodiment 2
[0031]
[0032] Add 4-bromothiophenol (0.5mmol) and tetra-n-butylammonium tetrafluoroborate (0.3mmol) to a 25mL reaction tube equipped with a magnetic stirrer, then add 6.0mL methanol; fix the reaction tube on a magnetic stirring Add electrodes (graphite cathode, graphite anode) to conduct 20mA constant current electrolysis, and at the same time stir the reaction solution evenly; after the mixture reacts at room temperature (25°C) for 6h, the reaction ends; the solvent is removed with a rotary evaporator, and the crude product is passed through the column Chromatography (petroleum ether: ethyl acetate = 20:1) separation and purification gave the target product (2b) with a yield of 86%. The NMR data of this compound are: 1 H NMR (600MHz, CDCl 3 )δ7.64(d, J=8.5Hz, 2H), 7.53(d, J=8.5Hz, 2H), 3.44(s, 3H). 13 C NMR (151MHz, CDCl 3 )δ142.74, 132.32, 127.12, 127.04, 49.94.
Embodiment 3
[0034]
[0035] Add 4-methylthiophenol (0.5mmol) and tetra-n-butylammonium tetrafluoroborate (0.3mmol) to a 25mL reaction tube equipped with a magnetic stirring bar, and then add 6.0mL methanol; fix the reaction tube on a magnetic On the stirrer, add electrodes (graphite cathode, graphite anode) to conduct 20mA constant current electrolysis, and at the same time stir the reaction solution evenly; after the mixture reacts at room temperature (25°C) for 6h, the reaction ends; the solvent is removed by a rotary evaporator, and the crude product is passed through The target product (2c) was obtained by separation and purification by column chromatography (petroleum ether: ethyl acetate = 20:1) with a yield of 76%. The NMR data of this compound are: 1 H NMR (600MHz, CDCl 3 )δ7.58(d, J=8.1Hz, 2H), 7.35–7.31(m, 2H), 3.46(s, 3H), 2.42(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ142.91, 140.81, 129.75, 125.40, 49.46, 21.53.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com