UDP-glycosyl transferases capable of catalyzing carbohydrate chain extension, and applications thereof
A technology of glycosyltransferase and glycosyl transfer, applied in the field of biotechnology and plant biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] Embodiment 1 Separation of glycosyltransferase and its coding gene
[0215] Ginseng RNA was extracted and reverse-transcribed to obtain ginseng cDNA. Using the cDNA as a template, use primer pair 1 (SEQ ID NO.:1 and SEQ ID NO.:2) or primer pair 2 (SEQ ID NO.:8 and SEQ ID NO.:9) to carry out PCR amplification to obtain 1.4- 1.5 kb amplification product. The DNA polymerase was selected from the high-fidelity KOD DNA polymerase of Treasure Bioengineering Co., Ltd. PCR products were detected by agarose gel electrophoresis.
[0216] Under UV irradiation, the target DNA band is excised. Then Axygen Gel Extraction Kit (AEYGEN Company) was used to recover DNA from the agarose gel, which was the amplified DNA fragment. The DNA fragment was ligated with the commercially available cloning vector pMD18-T Vector with the rTaq DNA polymerase of Treasure Bioengineering Co., Ltd. at the end, and the ligated product was transformed into commercially available Escherichia coli EPI300...
Embodiment 2
[0230] Example 2 Expression of Glycosyltransferase Gene GT29-32, GT29-33 and GT29-34 in Escherichia coli
[0231] Using the plasmids GT29-32-pMD18T, GT29-33-pMD18T and GT29-34-pMD18T constructed in Example 1 containing the GT29-32, GT29-33 and GT29-34 genes as templates, amplified with the primers shown in Table 1 Target genes GT29-32, GT29-33 and GT29-34.
[0232] After the expression vector pET28a (purchased from Merck) was digested with NcoI / SalI, GT29-32, GT29-33 and GT29-34 were cloned into pET28a (one-step cloning kit, purchased from Novizan), and Escherichia coli was constructed. Expression vectors GT29-32-pET28a, GT29-33-pET28a and GT29-34-pET28a. Using the 6×His tag sequence on pET28a, the C-terminals of the recombinant proteins GT29-32, GT29-33 and GT29-34 were tagged with 6×His tag. The plasmids were respectively transformed into commercially available E.coli BL21 to construct recombinant strains BL21-GT29-32, BL21-GT29-33 and BL21-GT29-34. Inoculate one recombin...
Embodiment 3
[0236] Embodiment 3GT29-32, the in vitro transglycosylation activity and product identification of GT29-33 and GT29-34
[0237]The supernatant of the cell lysate of recombinant Escherichia coli BL21-GT29-32, BL21-GT29-33 and BL21-GT29-34 in Example 2 was used as the crude enzyme solution to carry out the transglycosylation reaction, and the empty vector pET28a recombinant Escherichia coli Cell lysate served as a control.
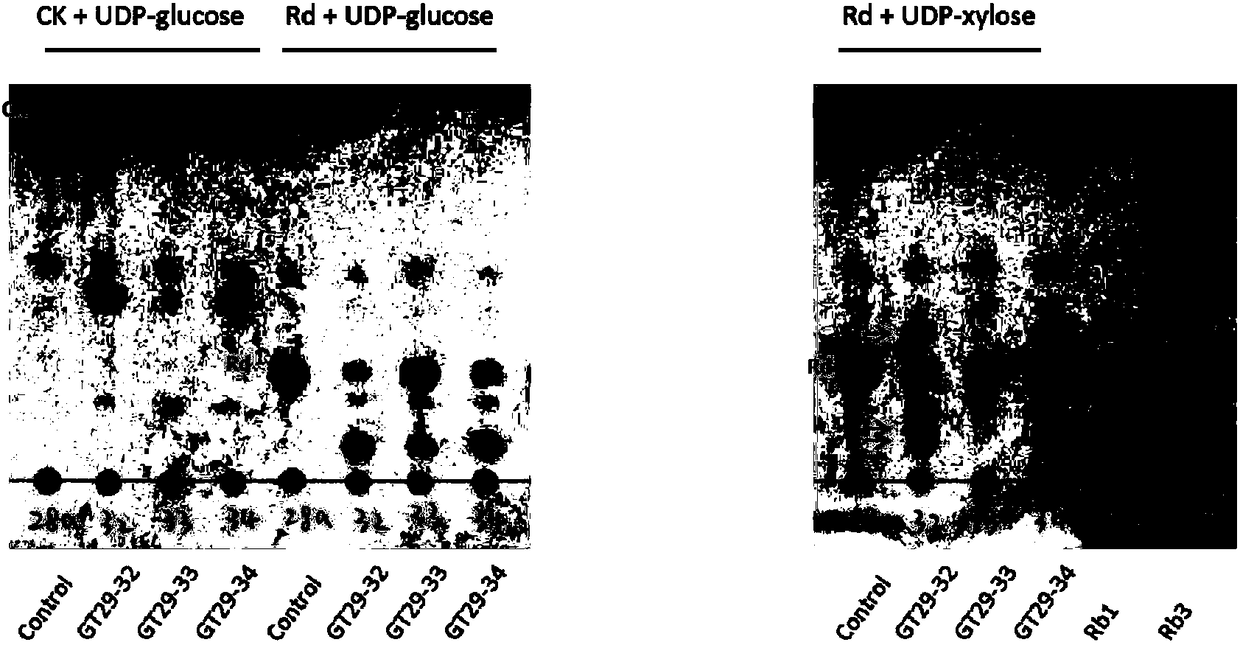
[0238] Such as figure 2 Shown: Protopanaxadiol-type ginsenoside CK is used as the glycosyl acceptor, UDP-glucose is used as the glycosyl donor, and GT29-32 and GT29-34 can catalyze it to generate a new product;
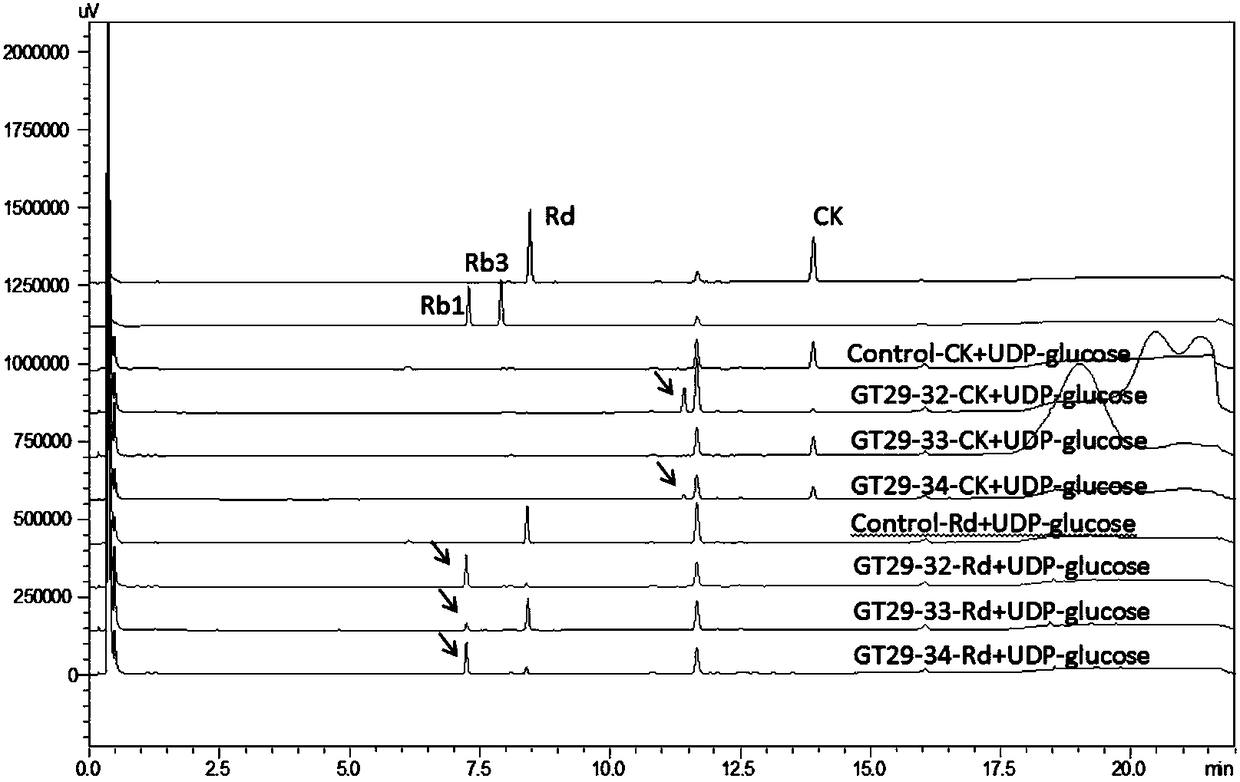
[0239] Such as image 3 Shown: With ginsenoside Rd as the glycosyl acceptor and UDP-glucose as the glycosyl donor, GT29-32, GT29-33 and GT29-34 can catalyze the formation of Rb1. The results of HPLC were consistent with those of TLC.
[0240] Therefore, GT29-32 and GT29-34 can catalyze C20-O-Glc of CK to extend a molecule of glucose to generate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com