A kind of cobalt phosphate nanomaterial and its preparation method and application
A cobalt phosphate nano and nano material technology, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of low current density, high overpotential, poor stability, etc., and achieve stable catalytic performance, small size, and reaction. mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 sample preparation
[0029] Mix the cobalt source, surfactant and water, stir for at least 1 hour, add phosphorus source aqueous solution after mixing, and stir for at least 2 hours to form an initial gel mixture, seal the initial gel mixture in 30 mL of Teflon-lined water Put it into a box-type resistance furnace in a thermal reaction kettle, crystallize at the crystallization temperature for a period of time, filter, wash, and dry to obtain purple flake Co 3 (OH) 2 (HPO 4 ) 2 nanomaterials. Table 1 shows the relationship between the types and ratios of raw materials in the initial gel mixture, crystallization temperature, crystallization time, and sample numbers.
[0030] Table 1 Relationship between sample synthesis conditions and sample numbers
[0031]
[0032]
Embodiment 2
[0033] The structural analysis of embodiment 2 sample
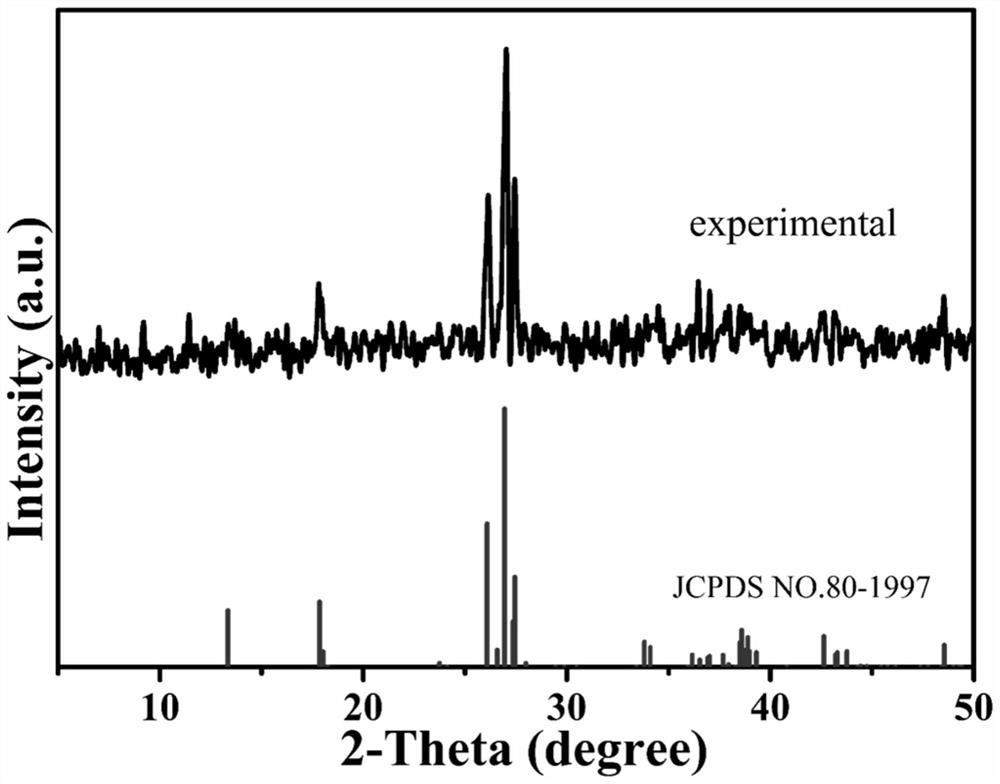
[0034] The powder X-ray diffraction method was used to analyze the structure of samples 1# to 10#.
[0035] Powder X-ray diffraction was carried out on the Miniflex II X-ray powder diffractometer of Japan RIGAKU Company. The test conditions were fixed target monochromatic light source Cu-Kα, wavelength The voltage and current are 30kV / 15A, the slits DivSlit / RecSlit / SctSlit are 1.25deg / 0.3mm / 1.25deg respectively, the scanning range is 5-50°, and the scanning step is 0.02°.
[0036] The results show that samples 1#~10# have the same chemical structural formula and crystal structure, and the chemical formula is Co 3 (OH) 2 (HPO 4 ) 2 . Take sample 1# as a typical representative, such as figure 1 shown with figure 2 In the spectrum obtained by X-ray diffraction test after the middle sample 1# is ground into powder, the peak position and peak intensity are consistent. It shows that the obtained samples are of high ...
Embodiment 3
[0037] Morphological characterization of embodiment 3 sample
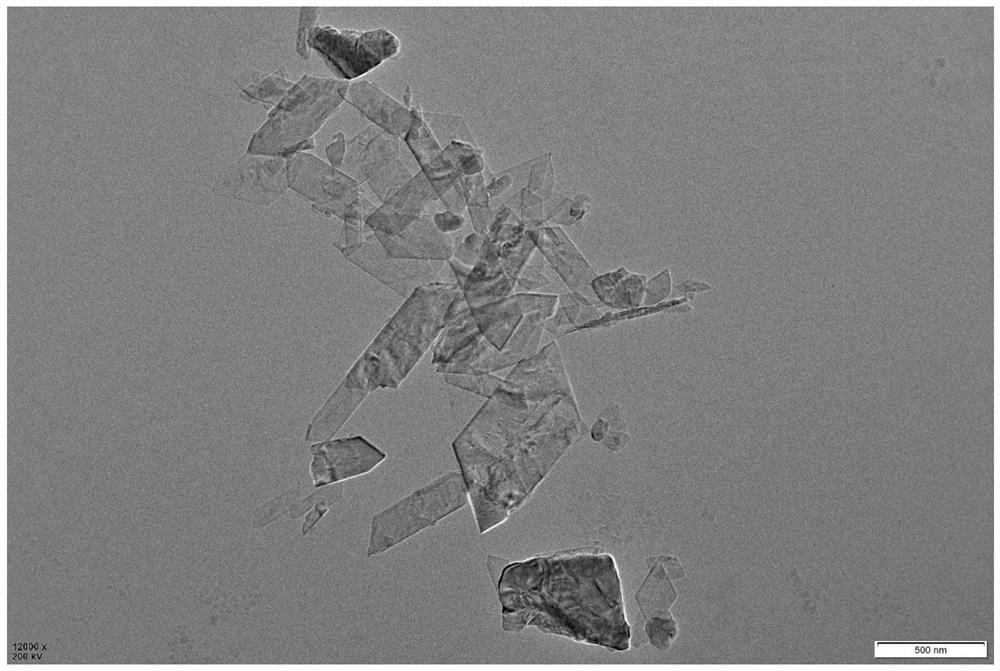
[0038] The morphology of sample 1# was characterized by scanning electron microscope and transmission electron microscope.
[0039] Scanning electron microscopy and transmission electron microscopy were carried out on a Hitachi S4800 scanning electron microscope and a JEOL JEM-2100F transmission electron microscope, respectively.
[0040] The result shows, as figure 2 SEM images and image 3 As shown in the transmission electron microscope, sample 1# has a good nanosheet shape, and the material has a uniform shape, good dispersion, and a size of 0.5-1 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com