Preparation method for Si-C-Si type anionic organosilicon surfactant
A surfactant, si-c-si technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
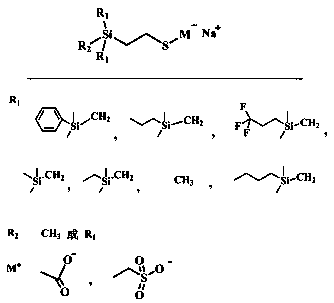
[0023] A kind of preparation method of Si-C-Si type anionic silicone surfactant, such as figure 1 Shown, this preparation method comprises the steps:
[0024] (1) Format response: in N 2 Under protection, chloromethylsilane is first prepared into Grignard reagent; then in N 2 Under protection, add vinylalkoxysilane or vinylchlorosilane into a four-necked flask filled with anhydrous tetrahydrofuran or anhydrous ether, and drop chloromethylsilane or methyl Grignard reagent to synthesize vinylsilicane;
[0025] (2) Mercapne reaction: put the vinylsilane obtained in (1) into a three-necked flask, add mercapto compound, solvent, and catalyst, and carry out mercaptoene reaction for 0.5~4 hours; extract with water-dichloromethane , the obtained solid was dried at 0.1 MPa and 40°C for 24 hours, and after cooling, a Si-C-Si type anionic silicone surfactant was obtained.
[0026] The chloromethylsilane is chloromethyl trimethylsilane, chloromethyl ethyl dimethyl silane, chloromethyl ...
Embodiment 1
[0035] (1) in N 2 Under protection, in a 250 ml four-neck flask, add 7.0 g methylvinyldichlorosilane to 50 ml anhydrous tetrahydrofuran, cool to 0 °C, add 8.0 g trimethylsilylmethylmagnesium chloride dropwise, and naturally rise to Stirring was continued at room temperature for 4 hours. After the reaction was completed, the reaction was terminated with anhydrous methanol, separated by filtration, extracted and separated, and concentrated to obtain vinyltrisilazane.
[0036] (2) In a 100ml three-necked flask equipped with a condenser, add 4.88 g of vinyl trisilane, 2.74 g of sodium thioglycolate, 10 ml of methanol, 0.16 g of benzoin dimethyl ether, N 2 The thioene reaction was carried out under protection for 30 minutes. After the reaction was completed, it was naturally cooled to room temperature, poured into 30 ml of ethyl acetate to precipitate the crude product, poured out the upper layer solution, and then added 2 ml of water to dissolve the crude product. Then extract wi...
Embodiment 2
[0038] (1) in N 2 Under protection, in a 250 ml four-neck flask, add 6.6 g of methylvinyldimethoxysilane to 50 ml of anhydrous ether, cool to 0 °C, add dropwise 8.0 g of trimethylsilylmethylmagnesium chloride, and naturally Warm up to room temperature and continue to stir for 5 hours. After the reaction is completed, the reaction is terminated with anhydrous methanol, separated by filtration, extracted and separated, and concentrated to obtain vinyltrisilane.
[0039] (2) In a 100ml three-necked flask equipped with a condenser, add 4.88 g of vinyl trisilane, 2.74 g of sodium thioglycolate, 10 ml of methanol, 0.16 g of benzoin dimethyl ether, N 2 Carry out the thioene reaction under protection for 30 minutes. After the reaction, naturally cool to room temperature, pour into 30 ml of ethyl acetate to precipitate the crude product, pour off the upper layer solution, add 2 ml of water to dissolve the crude product, and then use 8 ml of dichloro Methane extraction, repeated extrac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com