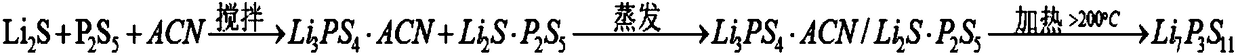Sulfide solid electrolyte and preparation method thereof and equipment
A solid-state electrolyte and sulfide technology, which is applied in circuits, electrical components, secondary batteries, etc., can solve problems affecting the performance of sulfide solid-state electrolytes, low reproducibility of sulfide solid-state electrolytes, and unsuitability for large-scale industrial production. , to achieve the effect of reducing the chance of raw materials in contact with water and air, reducing the production and preparation cycle, and eliminating the need for vacuum distillation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] A preparation method of a sulfide solid state electrolyte, the preparation method is that the reaction raw materials are placed in an anhydrous and oxygen-free sealed container to be ground, heated by microwave radiation for a certain period of time, and then cooled to obtain the sulfide solid state electrolyte.
[0041] Specifically, the reaction raw materials and abrasives are placed together in the sealed container, and the sealed container is filled with inert gas, such as argon, etc.; the sealed container is driven to move, so that the reaction raw materials are ground by the abrasives in the sealed container; When the sealed container is in a sealed state, the material in the sealed container is directly heated and reacted by microwave radiation for a period of time, and after cooling, a sulfide solid electrolyte is prepared. Preferably, the sealed container is a ball mill, the driving device is a planetary ball mill, and the rotation speed is set to be 100-3000rpm...
Embodiment 1
[0053] Take Li 2 S.P 2 S 5 As raw material, in an argon glove box with a molar ratio of 75:25 (Li 2 S:P 2 S 5 ) weighing 3.827gLi 2 S, and 6.164gP 2 S 5 , put it into the corundum ball mill jar, then add corundum crushed beads with a particle size of 3mm, and finally seal the ball mill jar.
[0054] The above-mentioned ball mill jar was placed in a ball mill, and mixed at a low speed of 800 rpm for 5 minutes, and then, the rotation speed was increased to 1800 rpm for 2 h. After completion, the ball mill jar was transferred to the microwave oven in the glove box, set under high heat conditions (power 1000W, frequency 2450MHz), microwave heating for 30 minutes. In the glove box, pass the material in the tank through a titanium mesh screen (16 mesh) to separate the corundum pulverized beads, and the obtained sulfide solid powder can be used to prepare a solid-state lithium battery electrolyte layer.
[0055] The lithium ion conductivity was measured by the AC impedance m...
Embodiment 2
[0057] Take Li 2 S.P 2 S 5 , LiCl as raw material, in an argon glove box with a molar ratio of 72:20:8 (LiCl 2 S:P 2 S 5 : LiCl) weighing 3.312gLi 2 S, 4.446gP 2 S 5 And 0.340g LiCl, put it into a teflon tank, then put zirconia crushed beads with a particle size of 3mm, and finally seal the teflon tank.
[0058] The above-mentioned polytetrafluoroethylene tank was placed in a ball mill, and crushed at a low speed of 800 rpm for 10 minutes, then, the rotation speed was increased to 1800 rpm, and mixed for 2 hours. The ball mill jar was transferred to a microwave oven in a glove box and heated for 30 minutes under high heat conditions (set power 800W, frequency 2450MHz). In the glove box, pass the material in the tank through a titanium mesh screen (16 mesh) to separate the corundum pulverized beads, and the obtained sulfide solid powder can be used to prepare a solid-state lithium battery electrolyte layer.
[0059] The lithium ion conductivity was measured by the AC i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com