A kind of preparation method of 5-hydroxymethylfurfural
A technology of hydroxymethyl furfural and 5-HMF, applied in the field of preparation of biological furfural, can solve the problems of high viscosity, long catalyst preparation time, long reaction time, etc., and achieves mild reaction conditions, good catalytic effect, and strong adjustable denaturation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
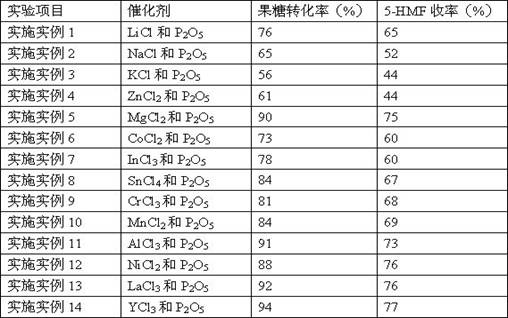
Embodiment 1~14
[0025] 60 mg of fructose was added to 1 mL of solvent DMSO with catalyst, some chloride and P 2 o 5 binary complexes of lithium chloride (LiCl), sodium chloride (NaCl), potassium chloride (KCl), zinc chloride (ZnCl) 2 ), magnesium chloride (MgCl 2 ), cobalt chloride (CoCl 2 ), indium chloride (InCl 3 ), tin chloride (SnCl 4 ), chromium chloride (CrCl 3 ), manganese chloride (MnCl 2 ), aluminum chloride (AlCl 3 ), nickel chloride (NiCl 2 ), lanthanum chloride (LaCl 3 ), yttrium chloride (YCl 3 ), the chloride and P in the catalyst 2 o 5 The molar ratio is 1:1, the chloride and P in the catalyst 2 o 5 The ratio of the total molar mass of fructose to the molar mass of fructose is 0.2:1, at 80 o The reaction was carried out at C for 30 min. After the reaction, the samples were tested by high-performance liquid chromatography, and the conversion rate of fructose and the yield of 5-HMF were calculated.
[0026] Table 1 Results of conversion of fructose to 5-HMF cataly...
Embodiment 15~17
[0029] With reference to the steps of implementation example 12, the difference is: NiCl 2 with P 2 o 5 The molar ratio is 0.5:1, 2:1, 3:1, NiCl in the catalyst 2 and P 2 o 5 The ratio of the total molar weight of the fructose to the molar weight of fructose is 0.15:1, 0.3:1, 0.4:1, repeat the above experiment, test the samples, and calculate the conversion rate of fructose and the yield of 5-HMF.
[0030] Table 2 Different NiCl 2 with P 2 o5 Effects of Compounds on Conversion of Fructose to 5-HMF
[0031]
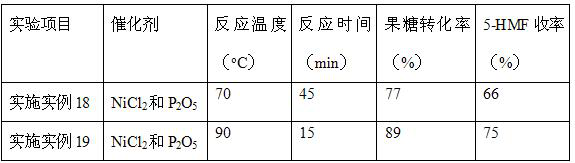
Embodiment 18~19
[0033] With reference to the step of embodiment example 12, difference is: when temperature of reaction is 70 o C, the reaction time is 45 min, when the reaction is 90 o C, the reaction time is 15 min, repeat the above experiment, test the samples, calculate the conversion rate of fructose and the yield of 5-HMF.
[0034] Table 3 Results of conversion of fructose to 5-HMF at different temperatures
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com