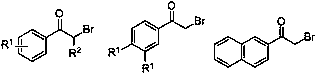
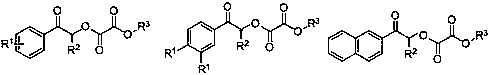
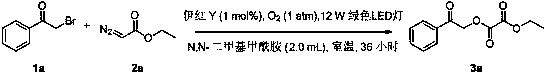Preparation method of oxalate
A technology of oxalate and -br, applied in the field of preparing oxalate, can solve the problem of no green method for synthesizing oxalate, and achieve the effects of easy functionalization, wide substrate versatility, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Add compound 1a (0.5 mmol, 101.6 mg), compound 2a (2 mmol, 224vL), Eosin Y (0.00 5mol, 3.6 mg), N,N-dimethylformamide into a 25mL Schlenk tube (2 mL); then react for 36 hours in oxygen under the irradiation of a 12W green LED lamp; after the reaction is complete, extract with ethyl acetate (10 mL × 3), dry with anhydrous magnesium sulfate, and remove the solvent with a rotary evaporator. The product 3a can be obtained by silica gel adsorption and simple column chromatography with a yield of 85%. The main test data of the prepared product are as follows. Through analysis, it can be known that the actual synthesized product is consistent with the theoretical analysis.
[0036] 1 H NMR (400 MHz, CDCl 3 ) δ 7.94 – 7.89 (m, 2H), 7.63 (t, J =7.4 Hz, 1H),7.50 (t, J =7.7 Hz, 2H), 5.55 (s, 2H), 4.40 (q, J =7.1 Hz, 2H), 1.40 (t, J =7.2Hz, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ 189.79, 156.91, 156.88, 134,10, 133.48,128.84, 127.65, 67.59, 63.35, 13.75; HRMS (ESI-TOF): Anal. ...
Embodiment 2
[0038]
[0039] Add compound 1b (0.5 mmol, 114.2 mg), compound 2a (2 mmol, 224vL), Eosin Y (0.005 mol, 3.6 mg), N,N-dimethylformamide into a 25mL Schlenk tube (2 mL). Then the system was reacted for 36 hours under the irradiation of a 12 W green LED lamp in oxygen. After the reaction, it was extracted with ethyl acetate (10 mL × 3), dried with anhydrous magnesium sulfate, and the solvent was removed with a rotary evaporator and silica gel was adsorbed. The product 3b was obtained by simple column chromatography with a yield of 73%. The main test data of the prepared product are as follows. Through analysis, it can be known that the actual synthesized product is consistent with the theoretical analysis.
[0040] 1 H NMR (400 MHz, CDCl 3 ) δ 8.03-7.97 (m, 1H), 7.66-7.59 (m, 1H), 7.33 --7.27 (m, 1H), 7.24-7.17 (m, 1H), 5.43 (d, J =3.7 Hz, 2H), 4.42 (q, J =7.1 Hz,2H), 1.42 (t, J =7.2 Hz, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ 188.00, 187.97, 163.65, 161.12, 157.04, 156.91, 136.10, 13...
Embodiment 3
[0042]
[0043] Add compound 1c (0.5 mmol, 119.5 mg), compound 2a (2 mmol, 224vL), Eosin Y (0.005 mol, 3.6 mg), N,N-dimethylformamide to a 25mL Schlenk tube (2 mL). Then the system was reacted for 36 hours under the irradiation of a 12 W green LED lamp in oxygen. After the reaction is complete, extract with ethyl acetate (10 mL × 3), dry with anhydrous magnesium sulfate, remove the solvent with a rotary evaporator, and adsorb on silica gel. The product 3c can be obtained by simple column chromatography with a yield of 90%. The main test data of the prepared product are as follows. Through analysis, it can be known that the actual synthesized product is consistent with the theoretical analysis.
[0044] 1 H NMR (400 MHz, CDCl 3 ) δ 7.50-7.36 (m, 3H), 7.18-7.13 (m, 1H), 5.52(s, 2H), 4.40 (q, J =7.1 Hz, 2H), 3.84 (s, 3H), 1.40 (s, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ 189.64, 159.82, 156.88, 134.69, 129.82, 120.47, 119.98, 111.95, 67.63, 63.29, 55.27, 13.71; HRMS (ESI-TOF): Anal. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com