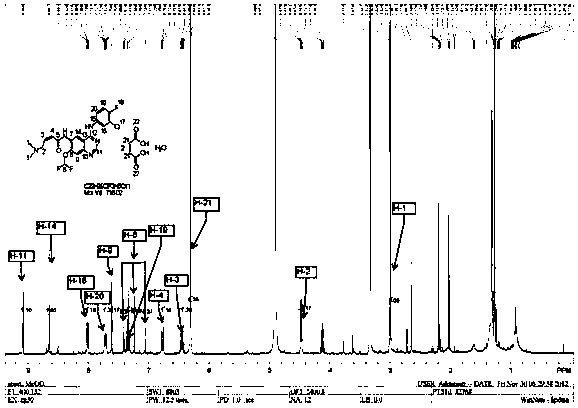
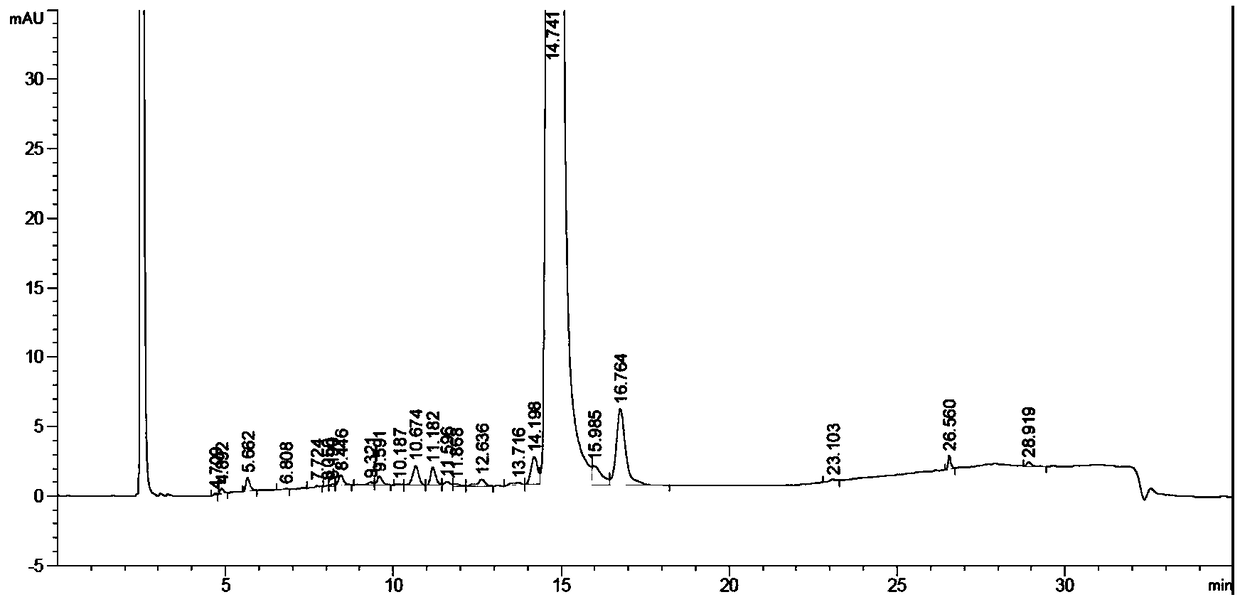
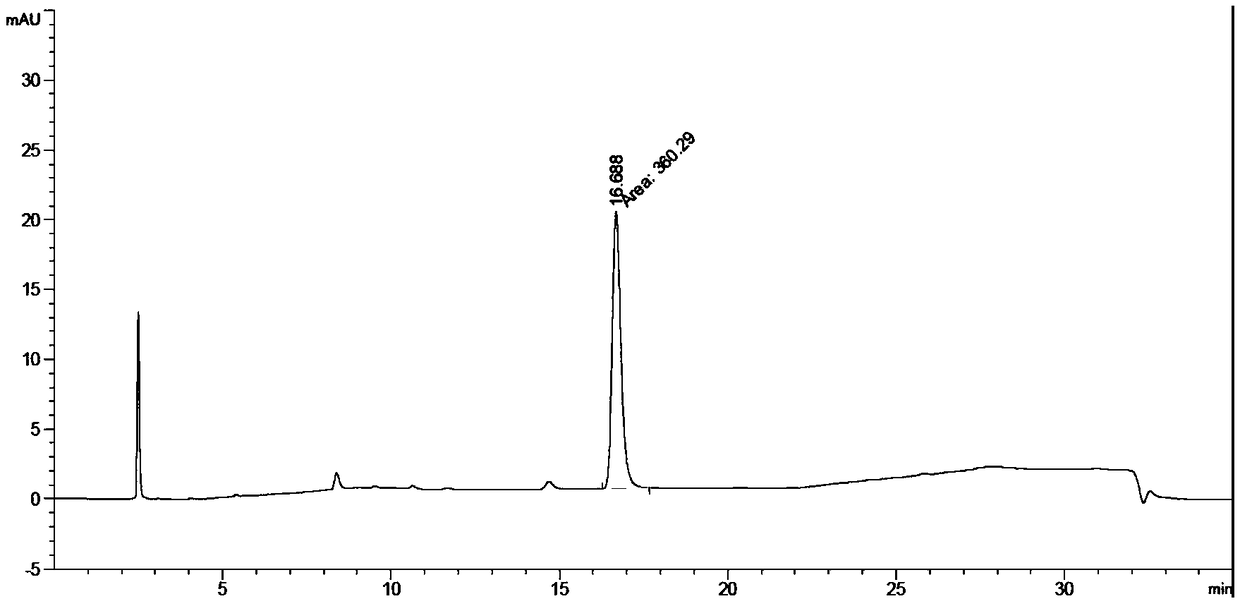
Mefatinib composition, related compound, and preparation method and application of Mefatinib composition
A composition and compound technology, applied in the directions of organic chemistry methods, drug combinations, carboxylate preparation, etc., can solve the problems of compound 1 that cannot be completely converted, difficult to cure, and has many impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1 meihuatinib composition
[0063] 1. Salt forming step
[0064] Add 479.7g of ethyl acetate and 812.6g of purified water into the reaction kettle, raise the temperature to about 50°C, then add 406.3g of A10 into the reaction kettle, stir to dissolve them all; add 203.1g of maleic acid to 1145.5g of acetic acid Add the ethyl acetate solution of maleic acid to the reaction kettle dropwise at 50°C, continue to stir for 1 hour after the dropwise addition, cool down naturally, stir and crystallize through liquid crystallization, filter, filter The cake was washed with 3.44Kg of ethyl acetate and dried under vacuum at 35°C to obtain the crude product of mevatinib.
[0065] 2. Recrystallization step
[0066] Add 10.51Kg of ethyl acetate and 1.05Kg of water into a 10L reaction kettle, raise the temperature to 50°C, add 525.7g of the above-prepared crude mevatinib, stir until fully dissolved and then lower to room temperature. Add 3.0 g meivatin...
Embodiment 2
[0069] Embodiment 2 Salt formation, crystallization condition
[0070] Referring to the salt-forming method in Example 1, the mass ratio of fixed ethyl acetate to water is about 10:1, and the amount of materials input and the results are shown in the table below.
[0071]
[0072] The results show that the amount of water added has a greater impact on the purity of the product and the content of the residual formula (II) compound in the product. Adding a lot of water is conducive to the promotion of product purity and the reduction of the content of the compound of formula (II), but if the amount of water added is too large, the compound of formula (II) will increase instead. In addition, the amount of water added also has an impact on the yield of the product, but the test found that stirring overnight can ensure that the yield is above 90%.
[0073] Referring to the recrystallization method of Example 1, the ratio of the crude product of meiwatinib, ethyl acetate and wat...
Embodiment 3
[0079] Synthesis of embodiment 3 formula (II) compound (cis isomer)
[0080]
[0081] The first step: the synthesis of 4-(dimethylamino)-2-yne butanoic acid
[0082] (At -78°C, n-butyllithium hexane solution (2.5M, 33.4mmol, 13.4ml) was slowly added dropwise to 1-dimethylamine-2-propyne (33.4mmol, 3.6ml, 2.78g) in dry tetrahydrofuran (15ml) solution. The resulting mixture was stirred at -78°C for 1 hour, crushed dry ice (335mmol, 11.72g) was added in one go, and stirred for 15 minutes. The reaction solution was poured into 300ml of water and washed with 100ml of ethyl acetate After washing three times, the aqueous phase was concentrated under reduced pressure. The resulting residue was dissolved in methanol, filtered to remove insoluble salts, and the filtrate was concentrated under reduced pressure to obtain 6.05 g of crude 4-(dimethylamino)-2-ynebutyric acid containing a large amount of inorganic salts.
[0083] MS (ESI) m / z = 128.2 [M+1].
[0084] The second step: the sy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com