Method for detecting ketoprofen in plasma by using high-performance liquid chromatography-tandem mass spectrometry and application
A technology of high-performance liquid chromatography and tandem mass spectrometry, which is applied in the field of detection of ketoprofen in plasma by high-performance liquid chromatography-tandem mass spectrometry, can solve the problems of high concentration of detection limit, large amount of sample required, and low sensitivity. The effect of low detection limit, fast analysis time, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] In a specific embodiment, the preparation method of ketoprofen standard solution comprises:
[0071] Accurately weigh 10.0 mg of ketoprofen standard substance, place it in a 10 mL brown volumetric flask, dissolve it with an appropriate amount of methanol, and then adjust the volume to the mark to prepare a 1.0 mg / mL ketoprofen standard solution.
[0072] The ketoprofen standard solution is stored at -20°C and is valid for 3 months.
[0073] In a preferred embodiment, the ketoprofen-D3 standard substance is dissolved in low-carbon alcohol to prepare a ketoprofen-D3 alcohol solution with a concentration of 0.1-2.0 mg / mL to obtain a ketoprofen internal standard solution.
[0074] Preferably, the concentration of ketoprofen-D3 alcohol solution is 0.5-1.5 mg / mL, for example, but not limited to 0.5 mg / mL, 0.8 mg / mL, 1.0 mg / mL, 1.2 mg / mL or 1.5 mg / mL mL, preferably 0.8-1.2 mg / mL.
[0075] By optimizing the concentration of ketoprofen-D3 alcohol solution, the optimization of ...
Embodiment 1
[0117] The present embodiment provides a method for detecting ketoprofen in plasma using high performance liquid chromatography-tandem mass spectrometry, comprising the following steps:
[0118] (a), the preparation of ketoprofen standard solution:
[0119] Accurately weigh 10.0mg of ketoprofen standard substance, place it in a 10mL brown volumetric flask, dissolve it with an appropriate amount of methanol, and then adjust the volume to the mark to prepare a 1.0mg / mL ketoprofen stock solution, and store it at -20°C for later use. Valid for 3 months.
[0120] (b), the preparation of ketoprofen internal standard solution:
[0121] Accurately weigh 10.05mg of ketoprofen-D3 standard substance into a 10mL brown volumetric flask, dissolve it with an appropriate amount of methanol, and then adjust the volume to the mark to obtain a 1mg / mL ketoprofen-D3 standard solution, and store it at -20°C for later use. Valid for 3 months.
[0122] (c), pretreatment of the sample to be tested:...
Embodiment 2
[0149] In this embodiment, the specificity of the method provided by the present invention for detecting ketoprofen in plasma by using high performance liquid chromatography-tandem mass spectrometry is determined:
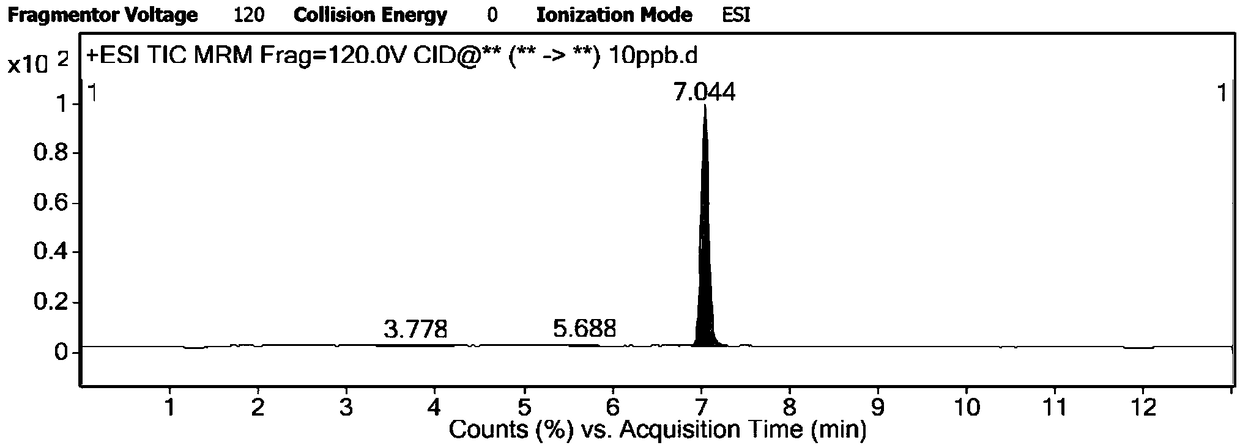
[0150] According to the EU Directive 2002 / 657 / EC, there should be no interfering peaks within 2.5% of the retention time deviation of the target substance in high performance liquid chromatography-tandem mass spectrometry analysis. This experiment analyzed the total ion chromatogram of the blank sample and matrix with a drug concentration of 10ng / mL, the results are as follows figure 1 , figure 2 It can be seen from the result figure that there is no interference peak in the blank sample, and the elution time of the drug added in the matrix is 7.04 min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com